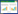急性肺损伤(acute lung injury, ALI)及其重症形式急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)为严重临床危象,机械通气虽为治疗核心,但不当设置可诱发气压伤、容积伤及肺组织周期性损伤,激发炎症反应、补体激活与氧化应激,增强肺毛细血管通透性及促凝血,加剧肺损伤病理过程,形成机械通气相关性肺损伤(ventilator induced lung injury,VILI),威胁患者氧合与肺功能[1]。
血管紧张素转化酶2(angiotensin-convertion enzyme 2, ACE2),系血管紧张素转换酶(angiotensin convertion enzyme, ACE)的同系物,血管紧张素(angiotensin, Ang)Ⅰ和Ang Ⅱ可分别在ACE2的作用下转化为Ang(1-9)、Ang(1-7),从而在肾素-血管紧张素系统(renin-angiotensin system, RAS)中发挥关键的抑制作用[2-3]。卡托普利(Captopril, CAP),作为一种经典的血管紧张素转化酶抑制剂(angiotensin converting enzyme inhibitor, ACEI),在心血管疾病的治疗中发挥着重要作用,其核心作用机制在于特异性地抑制体内ACE的催化活性,这一抑制效应显著减少AngⅠ向具有强烈缩血管及促增殖作用的AngⅡ的生物转化过程,可以抑制缓激肽降解酶的活性,促进血液中缓激肽水平的提升,进而对心血管系统起到保护作用[4]。研究显示ACE2/Ang(1-7)、ACE2/Ang(1-9)轴对肺、肾具有保护作用,减轻肺纤维化程度[5-7]。因此本研究探讨ACE2和CAP是否通过调控ACE2/Ang(1-7)/Ang(1-9)轴,发挥抗炎、改善内皮屏障等作用,减轻VILI。
1 材料与方法 1.1 实验动物本研究对象为72只体重18~22 g的SPF级4~6周龄雄性BALB/C小鼠,动物许可证号:SCXK(湘)2019-0004,购自湖南斯莱克景达实验有限公司。本研究经福建医科大学动物伦理委员会批准,批准号:IACUC FJMU 2023-Y-0438。12 h人工日光灯管照射,光照强度维持在200 Lux,室内环境温度调控在(23±1)℃的范围内,相对湿度保持在50%~80%,小鼠自由饮水和进食。
1.2 主要试剂及仪器小鼠血管性血友病因子(von willebrand factor,vWF)、内皮素1(Endothelin-1,ET-1)、可溶性细胞间粘附分子1(soluble intercellular adhesion molecule-1,sICAM-1)、血栓调节蛋白(thrombomodulin,TM)、血小板活化因子(platelet activating factor,PAF)、Ang(1-9)、Ang(1-7)、前列环素(prostacyclin,PG)I2、PGE2 ELISA检测试剂盒均购自上海酶联生物技术有限公司;兔ACE2多克隆抗体、鼠GAPDH单克隆抗体、辣根过氧化酶标记羊抗体兔IgG、辣根过氧化酶标记羊抗体鼠IgG购自成都正能生物技术有限责任公司;Genious 2X SYBR Green Fast qPCR Mix购自武汉爱博泰克生物科技有限公司;卡托普利购自上海麦克林生化科技有限公司。TransZol Up Plus RNA Kit和TransScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR(One-Step gDNA Removal)购自北京全式金生物技术股份有限公司。
主要仪器包括:化学发光仪购自上海勤翔科学仪器有限公司;KW-10小动物人工呼吸机购自南京卡尔文生物科技有限公司;荧光定量PCR仪和微量紫外-可见光分光光度计购自赛默飞世尔科技(中国)有限公司。
1.3 方法 1.3.1 模型建立及分组适应性喂养一周后,按照随机数字法把BALB/C小鼠分为6组,即正常对照组(normal control group,NC组)、VILI组、ACE2组、VILI+ACE2组、CAP组、VILI+CAP组,每组12只。腹腔内注射5%水合氯醛麻醉后,制备VILI模型采用大潮气量机械通气的方法[4]:仰卧位固定,颈部备皮、常规消毒,经口插管并连接呼吸机,设置潮气量为20 mL/kg,呼吸频率为60次/min,小鼠机械通气时间均为4 h。ACE2和CAP溶解于PBS液中,于机械通气前1 h,ACE2组和VILI+ACE2组腹腔内注射ACE2 0.1 mg/kg,CAP组和VILI+CAP组腹腔注射卡托普利2.5 mg/kg。NC组保留自主呼吸室内空气。
1.3.2 检测血清中血清vWF、ET-1、sICAM-1、TM、PAF、Ang(1-9)、Ang(1-7)、PGI2、PGE2的含量机械通气达到预定时间后,行心室内采血。在无菌环境中下,用组织剪开胸,使心脏和肺组织暴露,于心尖部采血,并快速分离血清于-80℃保存。使用ELISA试剂盒检测血清中vWF、ET-1、sICAM-1、TM、PAF、Ang(1-9)、Ang(1-7)、PGI2、PGE2的含量,严格按照说明书进行检测。
1.3.3 肺泡灌洗液中sICAM-1和总蛋白浓度的检测动物实验中,小鼠机械通气结束后,行支气管肺泡灌洗。参考郭君平等[8]的实验方法,收集小鼠支气管肺泡灌洗液(Bronchoalveolar lavage fluid, BALF)。用1 mL生理盐水灌洗气管3次,将灌洗液于4℃,3 000 g,离心10 min,上清液于-80℃冻存。使用ELISA试剂盒检测BALF中sICAM-1的含量,BCA试剂盒检测BALF中总蛋白的浓度。
1.3.4 肺组织湿/干重比(wet to dry, W/D)小鼠放血后取肺组织,每组取6只,滤吸干表面水分后,置于干净器皿内精确称量后,放于75℃恒温烤箱内,烘烤24 h至恒重后测定肺组织干重,计算W/D以评价肺组织水肿程度。
1.3.5 肺组织的苏木素-伊红染色(Hematoxylin and Eosin Staining, HE染色)及ELISA试剂盒检测指标取小鼠右侧肺组织,用组织固定液固定,乙醇梯度脱水,二甲苯透明、浸蜡,包埋制成蜡块,切成5 μm切片,进行HE染色,光镜下观察肺组织学形态及进行肺损伤评分。整取左侧肺部于液氮中速冻,之后放-80℃冰箱保存待检。称取肺组织0.1 g,制备成10%的组织匀浆,根据ELISA试剂盒的说明书检测肺组织中vWF、ET-1、sICAM-1、TM、PAF、Ang(1-9)、Ang(1-7)、PGI2、PGE2的水平。
1.3.6 实时荧光定量PCR(reverse transcription quantitative polymerase chain reaction RT-Qpcr,RT-qPCR)检测小鼠肺组织ACE2的相对表达量收集液氮速冻后的肺组织,使用RNA提取试剂盒提取总RNA,并根据反转录试剂盒的说明书进行反转录,得到cDNA。用稀释5倍的cDNA作为RT-qPCR的模板,使用ABI QuantStudio 3进行RT-qPCR。所用的引物序列为ACE2-F: 5'-GCAGATGGCTACAACTATAACCG-3',ACE2-R: 5'-CCTCCTCACATAGGCATGAAGA-3',GAPDH-F: 5'-TGGAAAGCTGTGGCGTGATG-3',GAPDH-R: 5'-TACTTGGCAGGTTTCTCCAGG-3';其中GAPDH作为内参基因。使用2-∆∆CT法计算结果。
1.3.7 Western blot测定ACE2蛋白的表达取-80℃冻存的肺组织,加入含蛋白酶抑制剂的高效组织裂解液RIPA,冰上反复吹打,12 000 g离心10 min后,取上清液用BCA法测蛋白浓度,制备样本;样本经12% SDS-PAGE分离蛋白后,转至PVDF膜上,5% BSA室温封闭1 h后,分别使用抗体ACE2(1∶1 000)、GAPDH(1∶5 000),4℃孵育过夜,TBST洗膜三次,加入HRP标记的羊抗鼠二抗(1∶5 000)或羊抗兔二抗(1∶5 000),室温孵育1 h,TBST洗膜三次,加入ECL化学发光液显色、曝光、拍照。使用Image J软件分析目的蛋白的灰度值。
1.4 统计学方法采用IBM SPSS 20.0统计软件进行数据分析。符合正态分布的计量资料以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,进一步两两比较采用LSD法。以P < 0.05为差异有统计学意义。
2 结果 2.1 HE染色结果NC、ACE2、CAP组肺脏实质为肺内支气管各级分支及其终末的大量肺泡,肺泡壁由单层上皮组成,间质包括肺内结缔组织及血管等,未见明显异常。与NC组比较,VILI组肺组织可见少量细支气管黏膜上皮细胞排列不规则,肺泡壁可见较多粒细胞浸润,肺泡大小不一,小范围肺泡壁毛细血管淤血;与VILI组相比,VILI+ACE1、VILI+CAP组肺损伤程度减轻,血管周围偶见散在的淋巴细胞浸润,少量血管淤血,肺泡壁可见少量粒细胞浸润,差异均有统计学意义(P < 0.05),见图 1A、1B。与NC组比较,VILI、VILI+ACE2、VILI+CAP组中的W/D比NC组的增加(P < 0.05);与VILI组比较,VILI+ACE2、VILI+CAP组中的蛋白W/D均显著下降(P < 0.05)。NC组与ACE2、CAP组中的W/D差异无统计学意义(P > 0.05),见图 1C。
|
| 注:A为肺组织病理改变(HE,×200);B为肺组织损伤评分;C为W/D比值;与NC组比较,aP < 0.05;与VILI组比较,bP < 0.05 图 1 各组小鼠肺组织病理学及W/D比值 Fig 1 Pathological changes and W/D ratio of lung tissues in mice of each group |
|
|
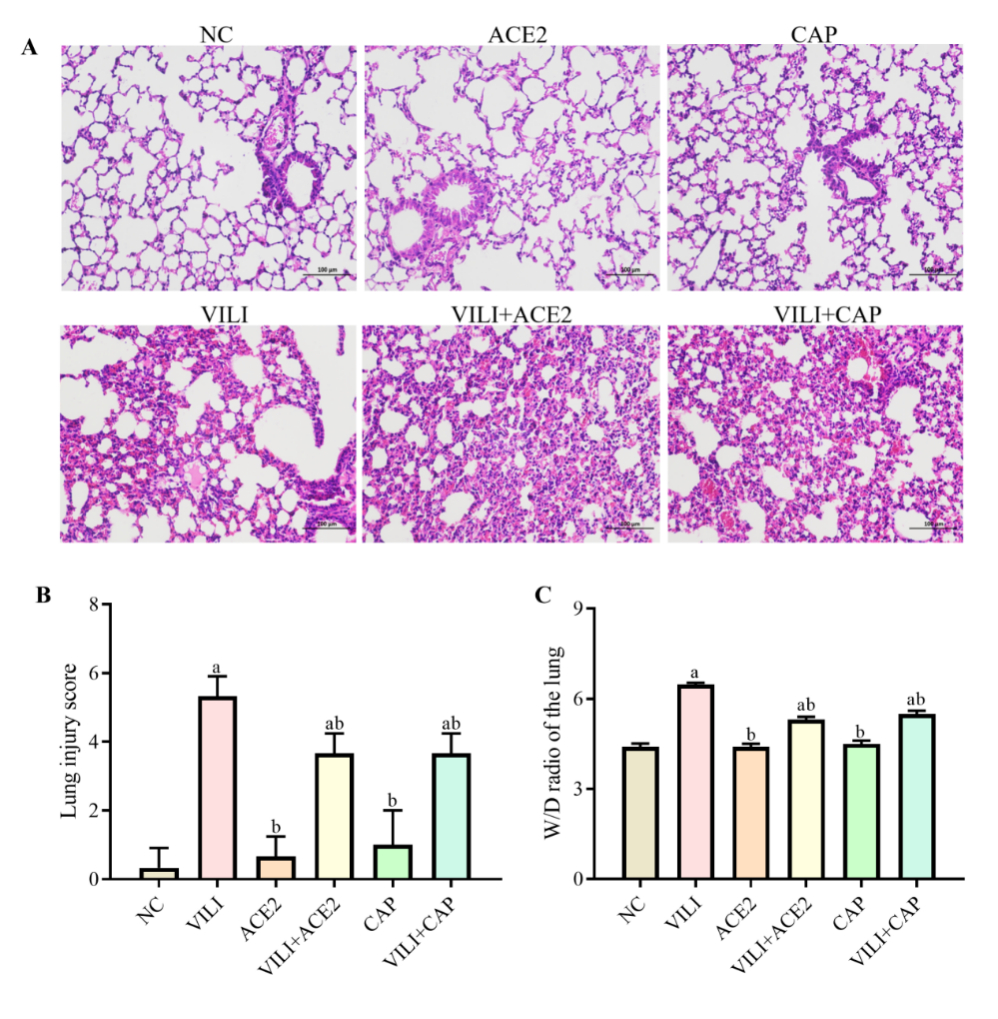
BCA法检测BALF中总蛋白和sICAM-1浓度,结果显示:与NC组比较,VILI、VILI+ACE2、VILI+CAP组中的总蛋白浓度均增加(P < 0.05);与VILI组比较,VILI+ACE2、VILI+CAP组中的总蛋白浓度均降低(P < 0.05),见图 2A。与NC组比较,VILI、VILI+ACE2、VILI+CAP组中的sICAM-1浓度均增加(P < 0.05);与VILI组比较,VILI+ACE2和VILI+CAP组中sICAM均降低(P < 0.05),见图 2B。AEC2、CAP组与NC组中的总蛋白、sICAM-1浓度均差异无统计学意义(P > 0.05)。
|
| 注:A为BALF中总蛋白的含量;B为BALF中sICAM-1的含量;与NC组比较,aP < 0.05;与VILI组比较,bP < 0.05 图 2 各组小鼠BALF中总蛋白和sICAM-1的浓度比较 Fig 2 Comparison of the concentrations of total protein and sICAM-1 in BALF of mice in each group |
|
|
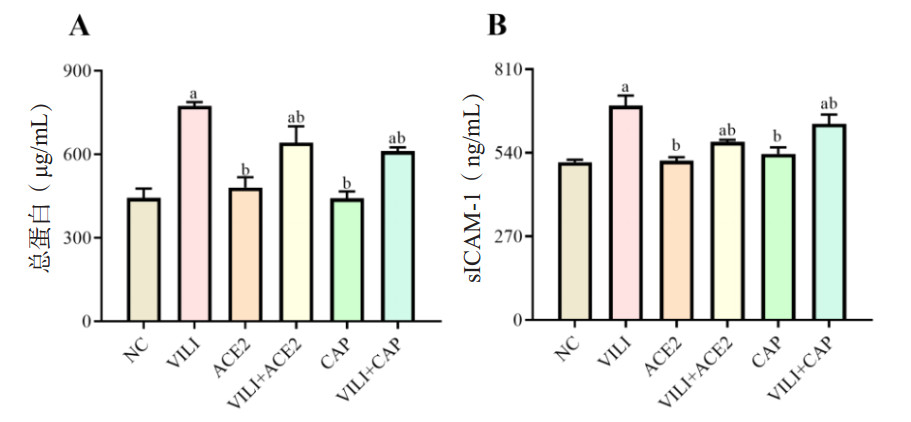
与NC组比较,VILI、VILI+ACE2、VILI+CAP组血清中PAF、ET-1、sICAM-1、vWF含量增加(P < 0.05);与VILI组比较,VILI+ACE2和VILI+CAP组中PAF、ET-1、sICAM-1、vWF均下降(P < 0.05)见图 3A~E。VILI组中的TM、Ang(1-9)、Ang(1-7)、PGI2含量比NC组下降(P < 0.05);与VILI组比较,VILI+ACE2和VILI+CAP组中的TM、Ang(1-7)、Ang(1-9)、PGI2的含量均增加(P < 0.05),见图 3F~I。所检测的这些指标,ACE2组、CAP组与NC组间均差异无统计学意义(P > 0.05)。
|
| 注:与NC组比较,aP < 0.05;与VILI组比较,bP < 0.05 图 3 各组小鼠血清中PAF、ET-1、sICAM-1、PGE2、vWF、TM、Ang(1-9)、Ang(1-7)及PGI2的表达水平 Fig 3 Expression levels of PAF, ET-1, sICAM-1, PGE2, vWF, TM, Ang(1-9), Ang(1-7) and PGI2 in serum of mice in each group |
|
|
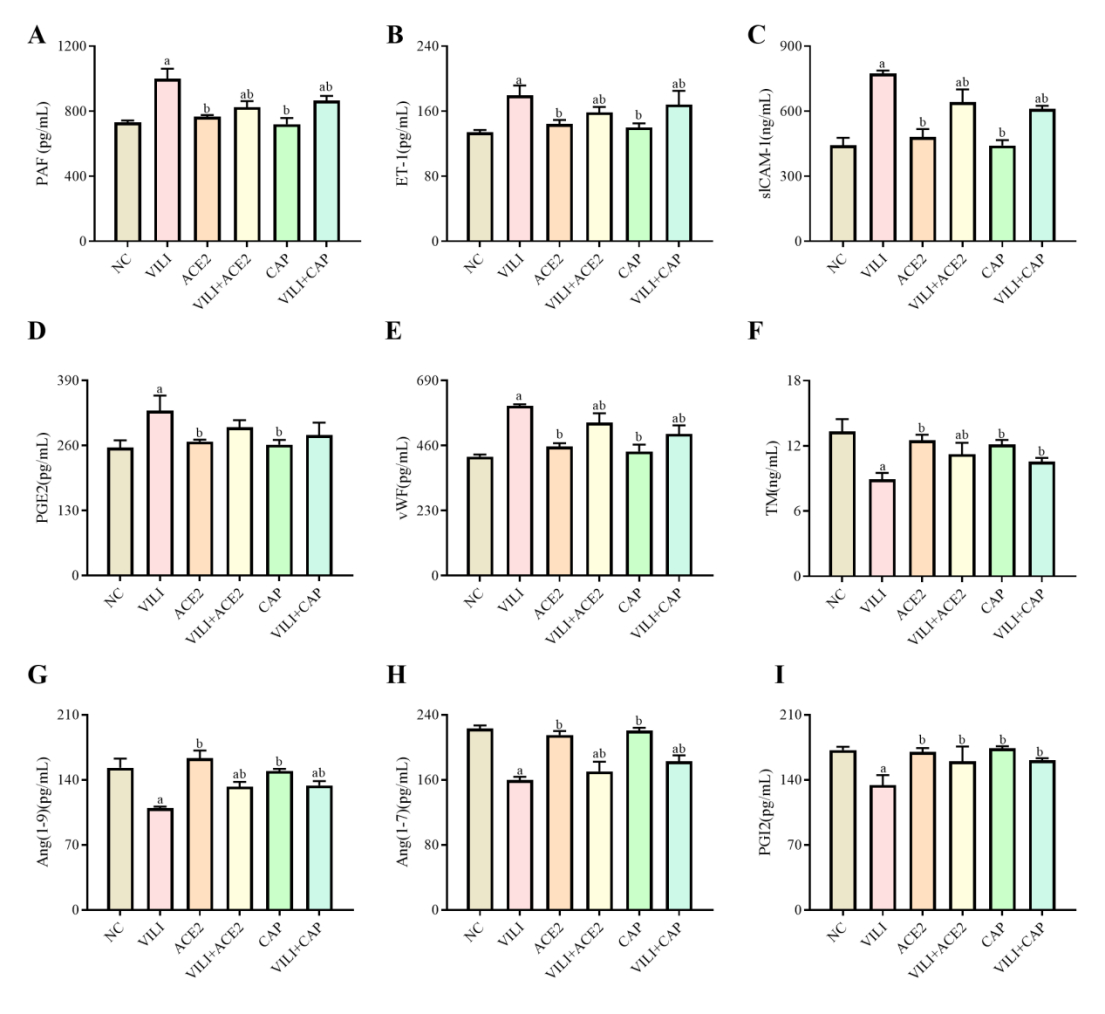
ELISA检测肺组织中PAF、ET-1、sICAM-1、PGE2、vWF、TM、Ang(1-9)、Ang(1-7)及PGI2的含量。结果显示:与NC组比较,VILI、VILI+ACE2、VILI+CAP组中PAF、ET-1、sICAM-1、vWF含量均增加(P < 0.05),VILI、VILI+ACE2组中PGE2的含量增加(P < 0.05),VILI+CAP组PGE2的含量差异无统计学意义(P > 0.05);与VILI组比较,VILI+ACE2和VILI+CAP组中PAF、ET-1、sICAM-1、PGE2、vWF均下降(P < 0.05),见图 4A~E。与NC组比较,VILI、VILI+ACE2、VILI+CAP组中的TM、Ang(1-9)、Ang(1-7)含量降低(P < 0.05),VILI组中PGI2含量降低(P < 0.05);与VILI比较,VILI+ACE2和VILI+CAP组中的TM、Ang(1-9)、Ang(1-7)、PGI2的含量均增加(P < 0.05),见图 4E~I。所检测的这些指标,ACE2、CAP与NC组间均差异无统计学意义(P > 0.05)。
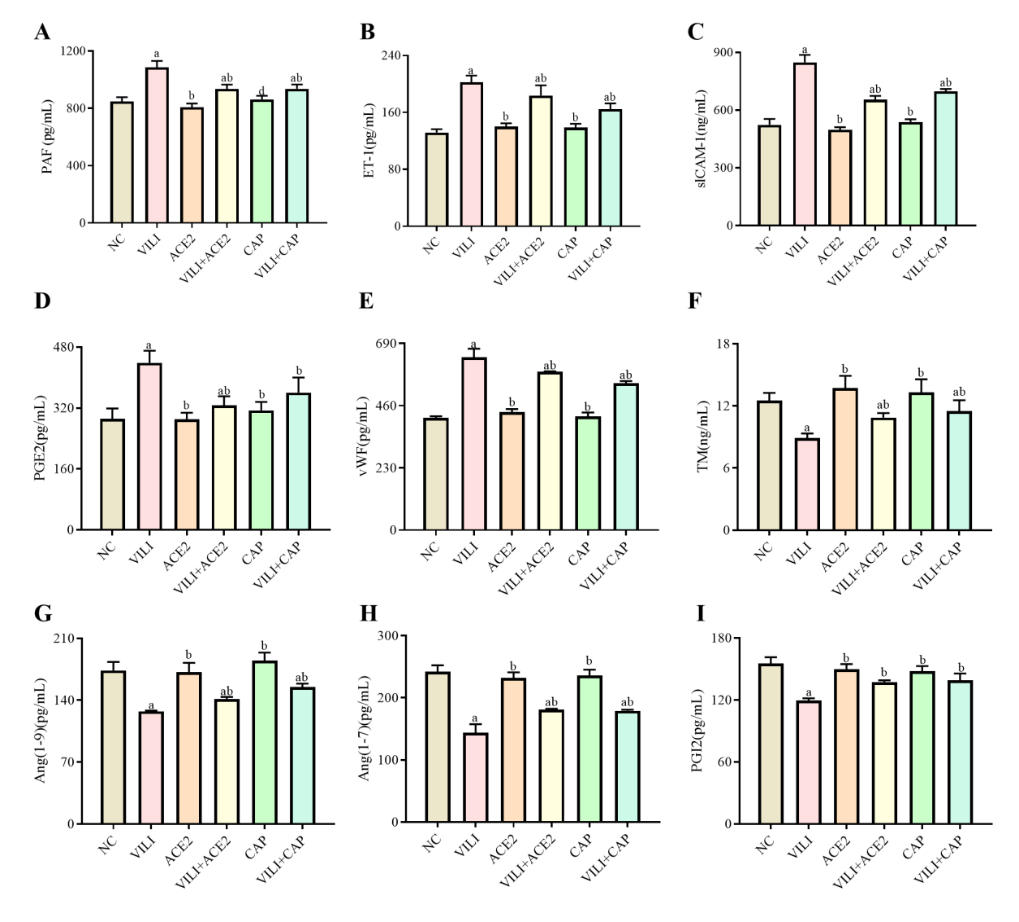
|
| 注:与NC组比较,aP < 0.05;与VILI组比较,bP < 0.05 图 4 各组小鼠肺组织中PAF、ET-1、sICAM-1、PGE2、vWF、TM、Ang(1-9)、Ang(1-7)及PGI2的表达水平 Fig 4 Expression levels of PAF, ET-1, sICAM-1, PGE2, vWF, TM, Ang (1-9), Ang (1-7), and PGI2 in lung tissue of mice in each group |
|
|
VILI组中ACE2蛋白和基因的水平都比NC组中的降低(P < 0.05),见图 5。和VILI比较,VILI+ACE2和VILI+CAP组中ACE2的蛋白和基因水平均增加(P < 0.05)。ACE2、CAP组与NC间差异无统计学意义(P > 0.05)。
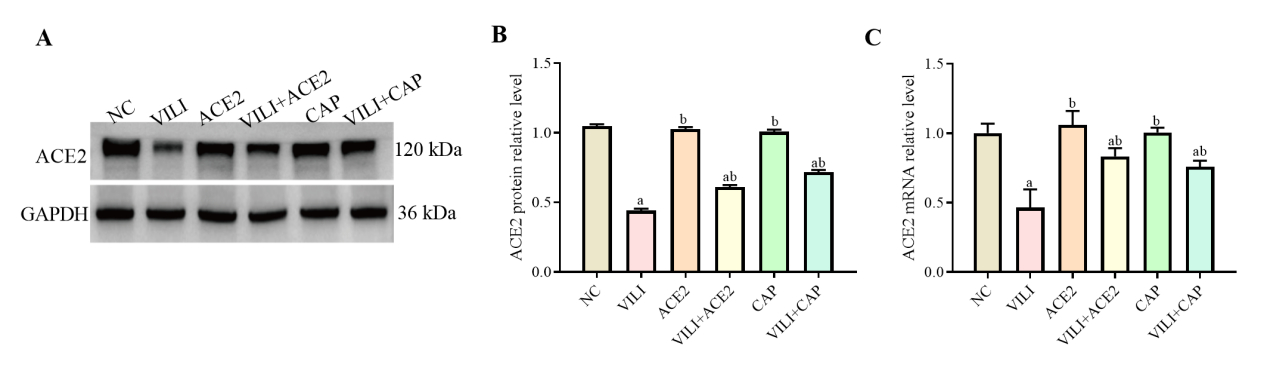
|
| 注:A为Western blot检测ACE2蛋白的结果;B为ACE2蛋白的相对表达量;C为RT-qPCR检测ACE2 mRNA表达情况;与NC组比较,aP < 0.05;与VILI组比较,bP < 0.05 图 5 各组小鼠肺组织中ACE2蛋白和基因的表达水平 Fig 5 Expression levels of ACE2 protein and mRNA in lung tissue of mice in each group |
|
|
ALI的主要症状是肺部出现剧烈的炎症反应,导致肺泡内皮屏障破坏、炎症细胞浸润,以及炎症和细胞毒介质的释放,肺泡上皮细胞和效应T细胞的活化[9]。大量炎症渗出使肺泡内充满富含蛋白质的液体,导致肺泡的呼吸功能受损,表面活性剂的合成和代谢受损进一步破坏患者的呼吸功能[10]。因大量炎症因子的破坏,肺组织的氧化与抗氧化之间的平衡也会遭到破坏[11]。本研究显示,腹腔注射ACE2或CAP,VILI小鼠肺组织和血清中炎症指标降低,心血管系统指标及肺组织病理形态有所改善,进一步促进肺功能的恢复。Xia等[12]和Boskabadi等[13]对ALI小鼠的研究结果表明,ACE2、CAP的促肺功能恢复作用被证实。
炎症是诱发肺部病理生理变化的关键因素[14]。研究表明炎症因子PAF[15]、ET-1[16]、sICAM-1[17]、PGE2[18]的水平与肺损伤密切相关。本实验结果显示,VILI小鼠PAF、ET-1、sICAM-1、PGE2水平较健康小鼠增加,在经过ACE2或CAP治疗后,上述炎性因子水平降低,提示ACE2或CAP具有抗炎作用,从而减轻炎症对VILI小鼠的肺损伤。Mungunsukh等[19]和Boskabadi等[20]的研究均表明CAP具有抑制肺损伤所引起的炎症反应的作用。Ye等[21]指出ACE2同样具有抗LPS诱导肺损伤引发的炎症效应。本研究与上述研究结果相似。
vWF是ALI肺内皮损伤的标志物[22],TM反映血管内皮损伤的严重程度[23]。PGI2能够减轻脂多糖诱导的ALI,并改善内皮屏障的恢复,降低炎症反应[24]。结果显示,VILI模型小鼠比健康小鼠血清和肺组织中TM、PGI2的水平降低,vWF水平增加;BALF中总蛋白和sICAM-1增加;在经过ACE2或者CAP干预后,血清中TM和PGI2的水平增加,vWF水平下降,BALF中总蛋白和sICAM-1降低,提示ACE2和CAP可以减少VILI的肺内皮细胞的损伤。研究已表明ACE2、CAP均具有改善内皮细胞的功能[25-26],本研究的结果与上述研究的结果相似。
ACE2和Ang(1-7)是心血管系统的关键保护因子,ACE2能够通过增进Ang(1-7)的生成,对ALI发挥保护功能[6]。Ang(1-9)可通过ACE或中性肽链内切酶的进一步水解作用,生成更多的Ang(1-7)[27]。ACE2水平降低会导致Ang(1-7)表达降低,从而致使Ang(1-7)与Mas受体结合后发挥的抗肺泡细胞凋亡、抗纤维化、抗炎等作用被削减[28-29],同时Ang Ⅱ的水平增加,促进炎症、纤维化等发展, 导致多器官病变、衰竭等[30]。本实验结果显示,VILI模型小鼠比健康小鼠血清和肺组织中Ang(1-7)、Ang(1-9)水平降低;在经过ACE2或者CAP干预后,血清和肺组织中Ang(1-7)、Ang(1-9)的水平增加。为了进一步证实ACE2和CAP对ACE2/Ang(1-9)/Ang(1-7)轴的调控,进一步检测各组肺组织中ACE2蛋白和mRNA的表达量。结果显示,经过ACE2或者CAP干预后,VILI模型小鼠肺组织中ACE2的蛋白和mRNA表达量均上升,提示ACE2和CAP可以通过调节ACE2/Ang(1-9)/Ang(1-7)轴发挥对VILI的保护作用。对于正常的小鼠,腹腔注射ACE2或CAP,所检测的指标均未发生显著变化,提示本实验所使用的ACE2或者CAP的剂量对正常小鼠没有显著的影响作用,且能对VILI发挥一定的保护作用。已有研究表明可以通过调节ACE2/Ang(1-7)/Mas轴降低炎症因子水平,改善肺损伤程度[31]。对于VILI模型小鼠,给予ACE2或者CAP,有助于抑制肺损伤,这与Li等[32]、Ye等[21]的研究结果一致,即CAP、ACE2一方面通过减轻炎症效应,另一方面通过增强ACE2的活力减轻肺损伤程度。
综上所述,腹腔注射ACE2或CAP均能降低VILI小鼠血清和肺组织中PAF、ET-1、sICAM-1、PGE2及vWF的水平,提高TM、Ang(1-9)、Ang(1-7)、PGI2的水平,ACE2蛋白和基因的表达水平显著增加,说明ACE2和CAP对VILI具有保护作用,ACE2和CAP均可以抑制炎症、保护心血管系统,ACE2和CAP可能是通过ACE2/Ang(1-9)/Ang(1-7)轴的作用,发挥对VILI的保护作用。VILI是一个复杂的过程,尚需要更多的研究证实ACE2、CAP对VILI的作用及相关机制。
利益冲突 所有作者声明无利益冲突
作者贡献声明 许俊平:论文撰写;陈霖、余天兴:研究设计、论文修改;张学平,谢万,张耿:实验操作、数据收集及整理;林新:提供研究经费、文章指导
| [1] | Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation[J]. Intensive Care Med, 2016, 42(5): 739-749. DOI:10.1007/s00134-016-4326-3 |
| [2] | Kittana N. Angiotensin-converting enzyme 2-angiotensin 1-7/1-9 system: novel promising targets for heart failure treatment[J]. Fundam Clin Pharmacol, 2018, 32(1): 14-25. DOI:10.1111/fcp.12318 |
| [3] | Bhushan S, Xiao ZW, Gao K, et al. Role and interaction between ACE1 ACE2 and their related genes in cardiovascular disorders[J]. Curr Probl Cardiol, 2023, 48(8): 101162. DOI:10.1016/j.cpcardiol.2022.101162 |
| [4] | Liu XH, Sun YT, Wei QL, et al. Captopril alleviates glucocorticoid-induced osteonecrosis of the femoral head by mediating the ACE2/Ang-(1-7)/Mas receptor cascade[J]. Eur J Pharmacol, 2022, 921: 174871. DOI:10.1016/j.ejphar.2022.174871 |
| [5] | Simões e Silva AC, Silveira KD, Ferreira AJ, et al. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis[J]. Br J Pharmacol, 2013, 169(3): 477-492. DOI:10.1111/bph.12159 |
| [6] | Liu L, Li Y, Li JX, et al. ACE2 expressed on myeloid cells alleviates sepsis-induced acute liver injury via the ang-(1-7)-mas receptor axis[J]. Inflammation, 2024, 47(3): 891-908. DOI:10.1007/s10753-023-01949-5 |
| [7] | Zhu XJ, Mou ZX, Han W, et al. All-trans retinoic acid inhibits oxidative stress via ACE2/Ang (1-7)/MasR pathway in renal tubular epithelial cells stimulated with high glucose[J]. Drug Dev Res, 2023, 84(5): 1008-1017. DOI:10.1002/ddr.22070 |
| [8] | 郭君平, 潘然, 王丽君, 等. 利拉鲁肽对脓毒症小鼠急性肺损伤的保护作用及其机制[J]. 中华急诊医学杂志, 2024, 33(8): 1134-1139. DOI:10.3760/cma.j.issn.1671-0282.2024.08.010 |
| [9] | Li WF, Zhao RQ, Wang XM, et al. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-κB pathway in vivo and vitro[J]. Inflammation, 2018, 41(3): 996-1007. DOI:10.1007/s10753-018-0753-3 |
| [10] | McVey MJ, Kapur R, Cserti-Gazdewich C, et al. Transfusion-related acute lung injury in the perioperative patient[J]. Anesthesiology, 2019, 131(3): 693-715. DOI:10.1097/ALN.0000000000002687 |
| [11] | Hu M, Yang JL, Xu Y. Effect of α-tocopherol in alleviating the lipopolysaccharide-induced acute lung injury via inhibiting nuclear factor kappa-B signaling pathways[J]. Bioengineered, 2022, 13(2): 3958-3968. DOI:10.1080/21655979.2022.2031399 |
| [12] | Xia XY, Wen CX, Cao WH, et al. Customized screening of ACE2 regulatory peptides from oyster to mitigate LPS-induced acute lung injury based on SPR technology[J]. Food Biosci, 2025, 68: 106754. DOI:10.1016/j.fbio.2025.106754 |
| [13] | Boskabadi J, Askari VR, Hosseini M, et al. Immunomodulatory properties of captopril, an ACE inhibitor, on LPS-induced lung inflammation and fibrosis as well as oxidative stress[J]. Inflammopharmacology, 2019, 27(3): 639-647. DOI:10.1007/s10787-018-0535-4 |
| [14] | Groves AM, Paris ND, Johnston CJ, et al. Mitigating viral impact on the radiation response of the lung[J]. Radiat Res, 2024, 202(3): 552-564. DOI:10.1667/RADE-24-00103.1 |
| [15] | Jiang T, Samapati R, Klassen S, et al. Mannose-6-phosphate attenuates acute lung injury by competitive release of acid sphingomyelinase from the mannose-6-phosphate receptor in endothelial caveolae[J]. Eur Respir J, 2025, 65(6): 2400003. DOI:10.1183/13993003.00003-2024 |
| [16] | Kurt A, Kalkan Y, Turut H, et al. Topiramate reduces aortic cross-clamping-induced lung injury in male rats[J]. Acta Medica (Hradec Kralove), 2018, 61(4): 144-149. DOI:10.14712/18059694.2018.133 |
| [17] | Yilmaz Y, Tumkaya L. Effects of hyperbaric oxygen and iloprost on intestinal ischemia-reperfusion induced acute lung injury[J]. Ann Surg Treat Res, 2019, 96(1): 34-40. DOI:10.4174/astr.2019.96.1.34 |
| [18] | Hezam K, Wang C, Fu EZ, et al. Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation[J]. Stem Cell Res Ther, 2023, 14(1): 48. DOI:10.1186/s13287-023-03277-9 |
| [19] | Mungunsukh O, George J, McCart EA, et al. Captopril reduces lung inflammation and accelerated senescence in response to thoracic radiation in mice[J]. J Radiat Res, 2021, 62(2): 236-248. DOI:10.1093/jrr/rraa142 |
| [20] | Boskabadi J, Mokhtari-Zaer A, Abareshi A, et al. The effect of captopril on lipopolysaccharide-induced lung inflammation[J]. Exp Lung Res, 2018, 44(4/5): 191-200. DOI:10.1080/01902148.2018.1473530 |
| [21] | Ye RS, Liu ZW. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway[J]. Exp Mol Pathol, 2020, 113: 104350. DOI:10.1016/j.yexmp.2019.104350 |
| [22] | El Basset Abo El Ezz AA, Abd El Hafez MA, El Amrousy DM, et al. The predictive value of Von Willebrand factor antigen plasma levels in children with acute lung injury[J]. Pediatr Pulmonol, 2017, 52(1): 91-97. DOI:10.1002/ppul.23518 |
| [23] | Watanabe-Kusunoki K, Nakazawa D, Ishizu A, et al. Thrombomodulin as a physiological modulator of intravascular injury[J]. Front Immunol, 2020, 11: 575890. DOI:10.3389/fimmu.2020.575890 |
| [24] | Toki S, Zhou WS, Goleniewska K, et al. Endogenous PGI2 signaling through IP inhibits neutrophilic lung inflammation in LPS-induced acute lung injury mice model[J]. Prostaglandins Other Lipid Mediat, 2018, 136: 33-43. DOI:10.1016/j.prostaglandins.2018.04.001 |
| [25] | He YX, Gang BC, Zhang MJ, et al. ACE2 improves endothelial cell function and reduces acute lung injury by downregulating FAK expression[J]. Int Immunopharmacol, 2024, 128: 111535. DOI:10.1016/j.intimp.2024.111535 |
| [26] | Wei JN, Xu H, Liu YY, et al. Effect of captopril on radiation-induced TGF-β1 secretion in EA.Hy926 human umbilical vein endothelial cells[J]. Oncotarget, 2017, 8(13): 20842-20850. DOI:10.18632/oncotarget.15356 |
| [27] | 郑梦迪, 王治. ACE2-Ang(1-7)-MasR轴对心血管系统保护作用的研究进展[J]. 中国心血管病研究, 2024, 22(6): 517-521. DOI:10.3969/j.issn.1672-5301.2024.06.003 |
| [28] | Pan H, Huang WH, Wang ZJ, et al. The ACE2-ang-(1-7)-mas axis modulates M1/M2 macrophage polarization to relieve CLP-induced inflammation via TLR4-mediated NF-кb and MAPK pathways[J]. J Inflamm Res, 2021, 14: 2045-2060. DOI:10.2147/JIR.S307801 |
| [29] | Abdel-Fattah MM, Elgendy ANAM, Mohamed WR. Xanthenone, ACE2 activator, counteracted gentamicin-induced nephrotoxicity in rats: Impact on oxidative stress and ACE2/Ang-(1-7) signaling[J]. Life Sci, 2021, 275: 119387. DOI:10.1016/j.lfs.2021.119387 |
| [30] | Wang CL, Ren LY, Chen SK, et al. Longdan Xiegan Tang attenuates liver injury and hepatic insulin resistance by regulating the angiotensin-converting enzyme 2/Ang (1-7)/Mas axis-mediated anti-inflammatory pathway in rats[J]. J Ethnopharmacol, 2021, 274: 114072. DOI:10.1016/j.jep.2021.114072 |
| [31] | Scozzi D, Liao FY, Krupnick AS, et al. The role of neutrophil extracellular traps in acute lung injury[J]. Front Immunol, 2022, 13: 953195. DOI:10.3389/fimmu.2022.953195 |
| [32] | Li YC, Zeng Z, Li YC, et al. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation[J]. Shock, 2015, 43(4): 395-404. DOI:10.1097/SHK.0000000000000302 |
 2025, Vol. 34
2025, Vol. 34