2. 首都医科大学附属北京朝阳医院急诊医学临床研究中心,北京 100020
2. Department of Emergency Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Beijing, 100020, China
脓毒症是住院患者死亡的主要原因之一[1-2],尤其是65岁及以上的老年患者,其病死率高达48.8%[3]。因此,早期识别出高死亡风险人群并实施个体化治疗对改善预后至关重要。脓毒症患者处于高分解代谢状态[4],且在入院初期即可出现急性肌肉减少[5]。床旁肌肉超声作为一种可靠、无创、便携且易于操作的肌肉量评估工具,已被广泛应用于临床[6]。研究表明,危重症患者的肌肉超声参数与躯体功能、住院时间、再入院率和生存率密切相关[7]。但老年脓毒症患者这一人群的肌肉变化情况及其对预后的影响鲜见报道。本研究旨在通过床旁超声评估老年脓毒症患者住院期间股四头肌厚度(quadriceps muscle layer thickness,QMLT)的动态变化,并探讨其对短期预后的预测价值。
1 资料与方法 1.1 研究对象本研究采用前瞻性、观察性研究方法,选取2024年7月1日至12月31日首都医科大学附属北京世纪坛医院急诊留观病区收治的老年脓毒症患者作为研究对象。纳入标准:(1)年龄≥65岁;(2)符合脓毒症3.0诊断标准[8]。排除标准:(1)患有恶病质或神经肌肉疾病的患者;(2)留观时间 < 7 d的患者;(3)无法配合完成超声检查的患者。
本研究严格遵守医学伦理学标准,并获得首都医科大学附属北京世纪坛医院伦理委员会批准(伦理号:IIT-2024-081-002)。所有受试者或其授权家属均签署知情同意书。
1.2 研究方法 1.2.1 临床资料收集记录患者的年龄、性别、体重指数(body mass index,BMI)、感染部位(包括呼吸系统、腹腔、泌尿系统、皮肤及软组织)。根据基础疾病情况计算合并症指数(Charlson comorbidity index,CCI)[9]。收集患者入院24 h内的实验室检查指标,若24 h内有多次检查,则选取最差值,包括白细胞计数(white blood cell,WBC)、红细胞计数(red blood cell,RBC)、血小板(platelet,PLT)、白蛋白(albumin,Alb)、C-反应蛋白(C-reactive protein,CRP),并计算序贯性器官衰竭(sequential organ failure assessment, SOFA)评分[8]。同时记录患者的住院时间(length of stay,LOS)。所有患者在住院期间均按照《拯救脓毒症运动:脓毒症与脓毒性休克治疗国际指南(2021)》接受标准治疗[10],包括液体复苏、抗菌药物使用、血管活性药物应用以及呼吸支持等。营养支持的目标是通过肠内和(或)肠外营养提供每日25~30 kcal/ kg的能量摄入及1.2~2.0 g/kg的蛋白质摄入[11]。
1.2.2 基于床旁超声动态评估股四头肌厚度在患者入院第1天,由一位具有床旁超声检查资质的急诊科医师使用便携式彩色超声诊断系统(通用电气医疗系统有限公司,中国,型号:Venue Fit Expert)及线阵探头(L4-12t RS)进行QMLT的测量,且该医师对患者预后保持未知。测量时,患者取仰卧位,下肢自然伸直并放松,探头横切放置于右髂前上棘与髌骨上缘之间中下1/3处,探头标记点朝向患者右侧[12]。未加压测量时,超声探头仅轻轻接触皮肤,不施加额外压力,并在探头上涂抹充足的耦合剂,以确保获得包含股骨皮质骨密度最高的图像,且无组织凹陷。加压测量则是在探头对下层组织施加最大压力后进行的QMLT测量[13]。为避免压力变化对测量结果的影响,首先进行未加压图像的采集与测量。测量完成后使用记号笔标记测量部位,并于患者入院第7天在同一部位重复测量未加压及加压QMLT。QMLT下降率的计算公式为:(第1天QMLT-第7天QMLT)/第1天QMLT×100%。
1.2.3 随访与分组通过电话随访受试者28 d的转归情况,并将其分为生存组和死亡组。
1.3 统计学方法采用SPSS 29.0统计软件及GraphPad Prism 9.4.1软件进行数据分析及作图。计量资料的正态性检验采用Shapiro-Wilk法,符合正态分布的数据以均数±标准差(x±s)表示,组间比较采用独立样本t检验;非正态分布的数据以中位数(下四分位数,上四分位数)[M(Q1, Q3)]表示,组间比较采用MannWhitney U检验。计数资料用例数(百分比)[n(%)]表示,组间比较采用χ2检验。组内两个时间点的QMLT比较采用Wilcoxon符号秩检验。采用单因素及多因素Cox回归分析QMLT动态变化对老年脓毒症患者短期预后的影响,分别绘制未加压及加压QMLT下降率预测患者28 d死亡风险的受试者工作特征(receiver operating characteristic, ROC)曲线,计算曲线下面积(area under curve,AUC),并根据最大约登指数确定QMLT下降率预测28 d病死率的最佳截断值,将患者分为高急性肌肉减少组和低急性肌肉减少组。采用Log-rank法进行生存比较,并绘制Kaplan–Meier曲线。以P < 0.05为差异有统计学意义。
2 结果 2.1 生存组与死亡组临床特征比较本研究共纳入137例老年脓毒症患者,其中生存组103例,死亡组34例,28 d病死率为24.8%。中位年龄为82(72,86)岁,男性63例(46.0%),女性74例(54.0%)。两组患者的年龄、性别、BMI、感染部位、WBC、RBC、PLT、CRP、LOS比较,差异无统计学意义(P均 > 0.05)。两组患者的CCI、Alb、SOFA评分比较,差异有统计学意义(P均 < 0.05),见表 1。
| 项目 | 总人数(137例) | 生存组(103例) | 死亡组(34例) | χ2/Z/t值 | P值 |
| 一般资料 | |||||
| 年龄[岁,M(Q1, Q3)] | 82(72,86) | 80(71,86) | 84(75,88) | 1.213 | 0.225 |
| 男性[例(%)] | 63(46.0) | 49(47.6) | 14(41.2) | 0.421 | 0.516 |
| BMI [kg/m2,x±s] | 23.06±4.20 | 22.97±4.19 | 23.35±4.27 | 0.462 | 0.645 |
| 感染部位[例(%)] | 1.617 | 0.656 | |||
| 呼吸系统 | 79(57.7) | 59(57.3) | 20(58.8) | ||
| 腹腔 | 37(27.0) | 27(26.2) | 10(29.4) | ||
| 泌尿系统 | 19(13.9) | 16(15.5) | 3(8.8) | ||
| 皮肤及软组织 | 2(1.5) | 1(1.0) | 1(2.9) | ||
| CCI[分,M(Q1, Q3)] | 6(5,7) | 5(4,7) | 6(5,8) | 2.107 | 0.035 |
| 实验室检查[M(Q1, Q3)] | |||||
| WBC(×109/L) | 9.74(7.16,14.66) | 9.61(6.90,14.73) | 10.14(8.14,14.71) | 0.571 | 0.568 |
| RBC(×1012/L) | 3.96(3.05,4.31) | 3.98(3.04,4.34) | 3.66(2.88,4.25) | -1.256 | 0.209 |
| PLT(×109/L) | 193(143,250) | 193(150,251) | 184(116,254) | -0.894 | 0.371 |
| Alb(g/L) | 36.0(31.8,39.9) | 36.8(33.1,40.8) | 34.2(29.1,39.1) | -2.464 | 0.014 |
| CRP(mg/L) | 49.0(9.5,99.5) | 42.0(9.0,95.0) | 67.7(10.8,141.9) | 1.184 | 0.237 |
| SOFA评分[分,M(Q1, Q3)] | 4(2,6) | 3(2,6) | 6(4,8) | 3.077 | 0.002 |
| LOS[天,M(Q1, Q3)] | 12(9,18) | 13(9,18) | 11(7,16) | -1.881 | 0.060 |
| 注:BMI体重指数;CCI合并症指数;WBC白细胞计数;RBC红细胞计数;PLT血小板计数;Alb白蛋白;CRP C-反应蛋白;SOFA评分序贯性器官衰竭评分;LOS住院时间 | |||||
对老年脓毒症患者入院第1天和第7天的未加压及加压QMLT进行组内比较,结果显示,无论是生存组还是死亡组,入院第7天的未加压QMLT(1.45 cm vs. 1.32 cm,P=0.035;1.37 cm vs. 1.20 cm,P < 0.001)及加压QMLT(0.95 cm vs. 0.89 cm,P=0.006;0.92 cm vs. 0.70 cm,P < 0.001)均低于第1天。结果表明,两组患者在入院第1周内均存在急性肌肉减少。
进一步对患者入院第1天和第7天的未加压及加压QMLT进行组间比较。结果显示,入院第1天时,生存组与死亡组患者的未加压QMLT(1.45 cm vs. 1.37 cm,P=0.932)及加压QMLT(0.95 cm vs. 0.92 cm,P=0.596)比较,差异均无统计学意义,表明两组患者在入院时的QMLT基线水平一致。入院第7天时,死亡组患者未加压QMLT(1.32 cm vs. 1.20 cm,P=0.006)及加压QMLT(0.89 cm vs. 0.70 cm,P=0.027)均低于生存组,见表 2。
| 时点 | QMLT(未加压) | QMLT(加压) | |||||||
| 生存组 | 死亡组 | Z值 | P值 | 生存组 | 死亡组 | Z值 | P值 | ||
| 第1天[cm,M(Q1, Q3)] | 1.45(1.14,1.82) | 1.37(1.13,1.86) | -0.085 | 0.932 | 0.95(0.71,1.13) | 0.92(0.61,1.18) | -0.531 | 0.596 | |
| 第7天[cm,M(Q1, Q3)] | 1.32(1.07,1.73) | 1.20(0.87,1.32) | -2.758 | 0.006 | 0.89(0.64,1.06) | 0.70(0.55,0.92) | -2.213 | 0.027 | |
| Z值 | -2.111 | -4.753 | -2.739 | -3.874 | |||||
| P值 | 0.035 | < 0.001 | 0.006 | < 0.001 | |||||
| 注:QMLT股四头肌厚度 | |||||||||
老年脓毒症患者入院第1周未加压及加压QMLT中位下降率分别为9.8%和10.4%。以是否发生28 d死亡为因变量,QMLT下降率为自变量,单因素Cox回归分析显示,死亡组患者未加压QMLT下降率高于生存组(19.7% vs. 7.1%,P < 0.001)。进一步将上述单因素分析中差异有统计学意义的变量纳入多因素Cox回归模型,校正CCI、Alb、SOFA评分后,未加压QMLT下降率仍是老年脓毒症患者28 d死亡的独立危险因素[校正后风险比(adjusted hazard ratio,aHR)=1.022,95%置信区间(confidence interval,CI):1.006~1.038,P=0.006],即未加压QMLT下降率每增加1%,28 d死亡风险增加2.2%,见表 3。
| QMLT下降率 | 总人数 | 生存组 | 死亡组 | 单因素分析 | 多因素分析a | |||||
| HR值 | 95% CI | P值 | aHR值 | 95% CI | P值 | |||||
| 未加压[%, M(Q1, Q3)] | 9.8(-9.3, 25.8) | 7.1(-16.3, 24.8) | 19.7(7.9, 30.3) | 1.029 | 1.012~1.045 | < 0.001 | 1.022 | 1.006~1.038 | 0.006 | |
| 加压[%, M(Q1, Q3)] | 10.4(-6.6, 22.8) | 6.5(-9.8, 21.8) | 17.1(4.1, 26.5) | 1.016 | 1.002~1.030 | 0.027 | 1.015 | 1.000~1.030 | 0.045 | |
| 注:a校正了CCI、白蛋白、SOFA评分,QMLT股四头肌厚度;HR值风险比;95% CI 95%置信区间;aHR校正后风险比。 | ||||||||||
同样地,单因素分析显示死亡组患者加压QMLT下降率高于生存组(17.1% vs. 6.5%,P=0.027),经多因素校正后,加压QMLT下降率仍是老年脓毒症患者28 d死亡的独立危险因素(aHR=1.015,95% CI:1.000~1.030,P=0.045),即加压QMLT下降率每增加1%,28 d死亡风险增加1.5%,见表 3。
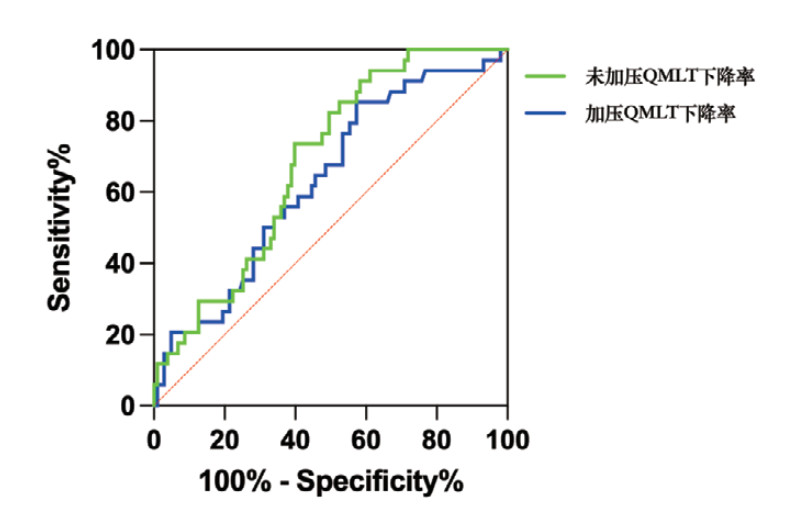
2.4 QMLT下降率对老年脓毒症患者28 d死亡的预测价值绘制QMLT下降率预测老年脓毒症患者28 d死亡的ROC曲线,见图 1。未加压QMLT下降率预测28 d死亡的AUC为0.683(95% CI: 0.591~0.776,P < 0.001),敏感度为73.5%,特异度为60.2%,最大约登指数为0.337,最佳截断值为10.55%。加压QMLT下降率预测28 d死亡的AUC为0.632(95% CI: 0.527~0.736,P=0.021),敏感度为85.3%,特异度为42.7%,最大约登指数为0.280,截断值为2.33%。DeLong非参数检验结果显示,未加压与加压QMLT下降率的预测效能差异无统计学意义(Z=1.170,P=0.242)。
|
| 图 1 QMLT下降率预测老年脓毒症患者28 d死亡的ROC曲线 Fig 1 The ROC Curves for predicting 28-day mortality in older patients with sepsis using QMLT decline rate |
|
|
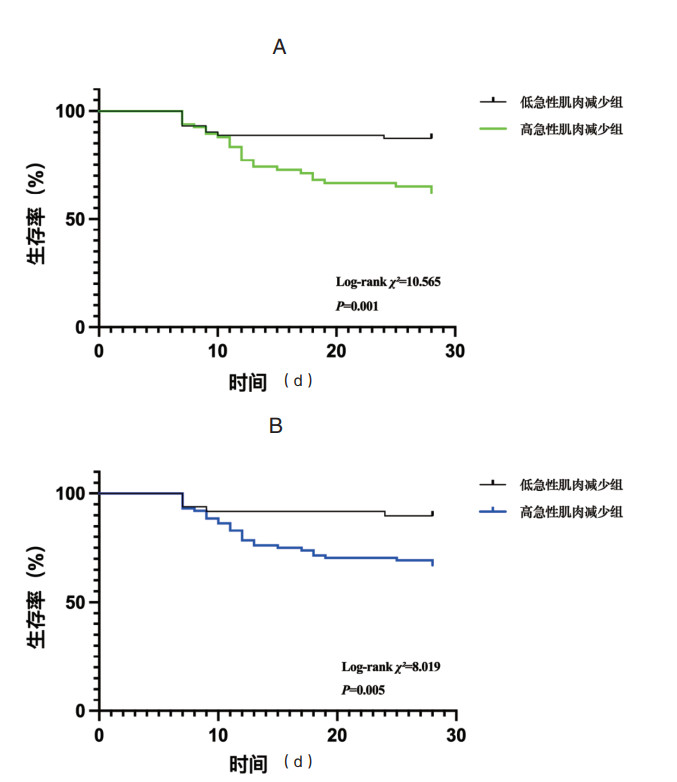
分别根据未加压及加压QMLT下降率的最佳截断值,将受试者分为高急性肌肉减少组和低急性肌肉减少组,Kaplan–Meier曲线显示,无论是未加压还是加压QMLT下降率评估的高急性肌肉减少组,其28 d死亡风险均高于低急性肌肉减少组(Log-rank χ2=10.565,P=0.001;Log-rank χ2=8.019,P=0.005),见图 2。
|
| 图 2 急性肌肉减少与老年脓毒症患者28 d死亡的的Kaplan–Meier曲线。a未加压QMLT下降率,b加压QMLT下降率 Fig 2 Kaplan-Meier curves for 28-day mortality in older septic patients with acute muscle loss. a QMLT decline rate obtained with minimum pressure, b QMLT decline rate obtained with maximum pressure |
|
|
近年来,床旁超声在危重症患者中的应用日益广泛。超声技术的普及使得肌肉量的连续监测成为可能,尤其是在资源有限的急诊科环境中,超声能够为临床医生提供肌肉的动态变化信息,有助于对老年脓毒症患者进行危险分层。股四头肌作为抗重力肌肉群,与躯体功能密切相关,且在仰卧位时易于触及,其厚度与全身肌肉量呈正相关。本研究通过床旁超声动态监测QMLT的变化,发现其在预测老年脓毒症患者短期预后方面具有较高的临床价值。
肌肉量在肌少症和营养不良的诊断中都是关键因素[14-15],而这两者都会增加不良结局的风险[16-17]。文献报道,重症监护病房(intensive care unit,ICU)的机械通气患者中,入院时基线肌肉量(如股直肌横截面积)越低,死亡风险越高[18]。而本研究结果发现,生存组与死亡组在入院时的QMLT基线水平差异无统计学意义,这可能与研究人群及测量指标的不同有关。
本研究发现,老年脓毒症患者在住院第1周内,无论是生存组还是死亡组,QMLT均显著下降,提示急性肌肉减少的发生。这一结果与既往研究一致[5]。Lopes等[18]报道,ICU患者在入院后第3天和第7天,QMLT分别减少了5%和13%。Pardo等[19]报道,ICU患者第1周QMLT下降率超过16%。本研究结果显示,老年脓毒症患者入院第1周未加压及加压QMLT中位下降率分别为9.8%和10.4%。这些数据表明,老年脓毒症患者在住院初期即面临较高的肌肉流失风险,这可能与炎症反应、代谢紊乱及营养摄入不足等因素有关。陈梦怡等[20]报道,股直肌厚度及横截面积的变异率与脓毒症患者的营养状况密切相关。
Lee等[21]报道,危重症患者入院的第1周内,QMLT每减少1%,60 d病死率增加5%。本研究表明,QMLT下降率是老年脓毒症患者28 d死亡的独立危险因素。未加压和加压QMLT下降率每增加1%,28 d死亡风险分别增加2.2%和1.5%。这一结果与胡庆河等[22]的研究一致,他们发现脓毒症患者QMLT的萎缩率是院内死亡的独立危险因素。本研究进一步证实,无论是未加压还是加压QMLT下降率,均能有效预测老年脓毒症患者的短期预后,且两者的预测效能差异无统计学意义。
在测量方法上,未加压测量避免了外部压力对肌肉形态的影响,能够更准确地反映肌肉的实际厚度,但在肥胖或皮下脂肪较厚的患者中,未加压测量可能受到脂肪层的干扰,影响测量精度。相比之下,加压测量通过压缩皮下脂肪层,能够更清晰地显示肌肉组织,减少脂肪对测量的干扰,但加压测量受操作者施加压力的影响较大,导致测量结果的可重复性较差。Fettcrplace等[23]分析了未加压及加压QMLT与CT图像中第3腰椎水平骨骼肌横截面积的关系,发现加压测量的QMLT能更好地反映骨骼肌横截面积。
本研究具有一定局限性。首先,本研究为单中心研究,样本量相对较小,可能存在选择偏倚。其次,本研究未将机械通气、肾脏替代治疗等干预措施纳入分析,而文献报道上述因素与急性肌肉减少密切相关[18, 24]。此外,本研究的观察期仅为1周,未来研究应进一步扩大样本量,纳入更多治疗相关变量,延长观察期并缩短评估间隔时间,并结合更多肌肉参数如横截面积、回声强度等,以更全面地评估肌肉超声在老年脓毒症患者中的应用价值。
综上,老年脓毒症患者在住院第1周存在急性肌肉减少,且QMLT下降率与短期预后密切相关。通过床旁超声连续监测QMLT的动态变化,有助于识别高死亡风险患者,从而为临床提供早期干预的依据。
利益冲突 所有作者声明不存在利益冲突
作者贡献声明 李秋敬:实验设计、数据收集、统计分析、论文撰写;商娜:实验设计、论文修订;陈必耀:研究实施、数据收集与整理;杨铁城:统计分析、论文审校、质量控制;所有作者确认了论文的最终稿
| [1] | Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study[J]. Lancet, 2020, 395(10219): 200-211. DOI:10.1016/S0140-6736(19)32989-7 |
| [2] | Rhee C, Jones TM, Hamad Y, et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals[J]. JAMA Netw Open, 2019, 2(2): e187571. DOI:10.1001/jamanetworkopen.2018.7571 |
| [3] | Martin-Loeches I, Guia MC, Vallecoccia MS, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: a prospective, observational, multicenter cohort study[J]. Ann Intensive Care, 2019, 9(1): 26. DOI:10.1186/s13613-019-0495-x |
| [4] | 刘红升, 张庆红. GLP-1在脓毒症持续性炎症反应-免疫抑制-分解代谢综合征中的作用机制[J]. 中华急诊医学杂志, 2023, 32(12): 1737-41. DOI:10.3760/cma.j.issn.1671-0282.2023.12.030 |
| [5] | Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness[J]. JAMA, 2013, 310(15): 1591-1600. DOI:10.1001/jama.2013.278481 |
| [6] | Nijholt W, Scafoglieri A, Jager-Wittenaar H, et al. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review[J]. J Cachexia Sarcopenia Muscle, 2017, 8(5): 702-712. DOI:10.1002/jcsm.12210 |
| [7] | Casey P, Alasmar M, McLaughlin J, et al. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: a systematic review[J]. J Cachexia Sarcopenia Muscle, 2022, 13(5): 2298-2309. DOI:10.1002/jcsm.13041 |
| [8] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [9] | Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation[J]. J Chronic Dis, 1987, 40(5): 373-383. DOI:10.1016/0021-9681(87)90171-8 |
| [10] | Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021[J]. Intensive Care Med, 2021, 47(11): 1181-1247. DOI:10.1007/s00134-021-06506-y |
| [11] | McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.)[J]. JPEN J Parenter Enteral Nutr, 2016, 40(2): 159-211. DOI:10.1177/0148607115621863 |
| [12] | Palakshappa JA, Reilly JP, Schweickert WD, et al. Quantitative peripheral muscle ultrasound in sepsis: Muscle area superior to thickness[J]. J Crit Care, 2018, 47: 324-330. DOI:10.1016/j.jcrc.2018.04.003 |
| [13] | Paris MT, Lafleur B, Dubin JA, et al. Development of a bedside viable ultrasound protocol to quantify appendicular lean tissue mass[J]. J Cachexia Sarcopenia Muscle, 2017, 8(5): 713-726. DOI:10.1002/jcsm.12213 |
| [14] | Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis[J]. Age Ageing, 2019, 48(1): 16-31. DOI:10.1093/ageing/afy169 |
| [15] | Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community[J]. Clin Nutr, 2019, 38(1): 1-9. DOI:10.1016/j.clnu.2018.08.002 |
| [16] | Zhang XM, Chen DH, Xie XH, et al. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: a systematic review and meta-analysis[J]. BMC Geriatr, 2021, 21(1): 339. DOI:10.1186/s12877-021-02276-w |
| [17] | Milanez DSJ, Razzera EL, Lima J, et al. Feasibility and criterion validity of the GLIM criteria in the critically ill: a prospective cohort study[J]. JPEN J Parenter Enteral Nutr, 2023, 47(6): 754-765. DOI:10.1002/jpen.2536 |
| [18] | Lopes MLG, Cidade JP, Sousa D, et al. Ultrasound assessment of muscle mass in critically ill patients: a correlation with nutritional support and clinical outcomes[J]. J Crit Care, 2025, 85: 154938. DOI:10.1016/j.jcrc.2024.154938 |
| [19] | Pardo E, El Behi H, Boizeau P, et al. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients[J]. BMC Anesthesiol, 2018, 18(1): 205. DOI:10.1186/s12871-018-0647-9 |
| [20] | 陈梦怡, 姜煜浩, 冯辉, 等. 股直肌肌肉超声在评估脓毒症患者营养状况中的作用[J]. 中华急诊医学杂志, 2025, 34(10): 1382-9. DOI:10.3760/cma.j.cn114656-20250309-00170 |
| [21] | Lee ZY, Ong SP, Ng CC, et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: a single-center prospective observational study[J]. Clin Nutr, 2021, 40(3): 1338-1347. DOI:10.1016/j.clnu.2020.08.022 |
| [22] | 胡庆河, 孙鹏, 张春灵, 等. 早期床旁超声测量脓毒症患者股四头肌变化对住院死亡的诊断价值[J]. 中华危重病急救医学, 2022, 34(10): 1060-5. DOI:10.3760/cma.j.cn121430-20220822-00773 |
| [23] | Fetterplace K, Corlette L, Ali Abdelhamid Y, et al. Assessment of muscle mass using ultrasound with minimal versus maximal pressure compared with computed tomography in critically ill adult patients[J]. Aust Crit Care, 2021, 34(4): 303-310. DOI:10.1016/j.aucc.2020.10.008 |
| [24] | Teixeira JP, Griffin BR, Pal CA, et al. Critical illness myopathy and trajectory of recovery in acute kidney injury requiring continuous renal replacement therapy: a prospective observational trial protocol[J]. BMJ Open, 2023, 13(5): e072448. DOI:10.1136/bmjopen-2023-072448 |
 2025, Vol. 34
2025, Vol. 34