2. 西安交通大学第二附属医院, 西安 710038
Sepsis-3.0定义脓毒症为宿主对感染反应失调而导致的危及生命的器官功能障碍,其诊断依赖于脓毒症相关序贯器官衰竭评估(sequential organ failure assessment, SOFA)评分,感染发生后SOFA评分较基线升高≥2分,即可临床诊断为脓毒症。该定义突出了器官功能障碍在脓毒症中的核心地位。乳酸作为代谢紊乱的关键指标,不仅是组织低灌注的标志,更直接反映了线粒体功能障碍和免疫代谢重塑[1]。尽管脓毒症治疗方面已取得一定进展,患者病死率仍居高不下,因此深入探索其器官损伤的机制、寻找新的治疗靶点具有重要意义[2-3]。近年来研究发现,乳酸除参与能量代谢外,还可通过介导蛋白质乳酸化修饰,广泛参与脓毒症中的表观遗传调控及多器官损伤进程[4]。本综述系统阐述乳酸化修饰在脓毒症器官损伤中的核心作用,揭示代谢重编程驱动免疫失衡与器官功能障碍的新机制,以期为脓毒症的早期诊断、预后评估及靶向治疗提供新思路。
1 乳酸:从代谢产物到信号分子近年研究表明,乳酸远非传统认为的代谢废物,而是具备多重生物学功能的信号分子。乳酸可特异度结合G蛋白偶联受体81(G-protein-coupled receptor 81, GPR81),该受体具有典型七次跨膜结构域,乳酸结合后可激活G蛋白下游信号,降低细胞内环磷酸腺苷(cyclic adenosine monophosphate, cAMP)水平,抑制脂肪分解[5]。此外,GPR81还能感知细胞外乳酸浓度,调控乳酸代谢相关基因的表达,增强细胞对乳酸的摄取,并促进线粒体生物发生关键调控因子PGC-1α的表达,推动乳酸进入线粒体进行氧化代谢,促进线粒体网络重建与呼吸链复合体增殖。这一过程不仅维持细胞内pH稳态,还通过乳酸的有效清除与能量转化,支持细胞在缺氧或高代谢状态下的适应性反应[6]。
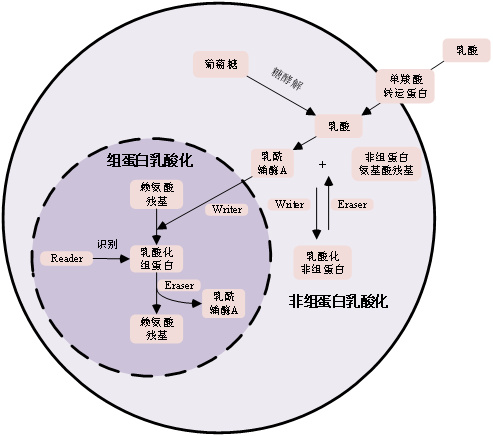
2 乳酸化修饰的分子机制乳酸化修饰是一种新兴的蛋白质翻译后修饰方式,其机制如图 1所示。巨噬细胞通过糖酵解产生内源性乳酸,缺氧诱导因子1α(hypoxia-inducible factor 1-α,HIF-1α)上调单羧酸转运蛋白(monocarboxylate transporter, MCT)表达,促进乳酸释放,从而激活“乳酸时钟”,启动赖氨酸乳酸化修饰。乳酸在细胞内转化为乳酰辅酶A,进而由“writer”类酶(如组蛋白乙酰转移酶p300/CBP)催化,将其转移至靶蛋白特定赖氨酸残基,改变蛋白构象与功能,影响基因转录[7-8]。另一方面,“eraser”酶负责去乳酸化修饰,以动态调控该过程;而“reader”蛋白则识别这些修饰并介导下游生物学效应。
|
| 图 1 蛋白质乳酸化修饰机制示意图 |
|
|
乳酸化修饰不仅发生于组蛋白,也广泛存在于非组蛋白。例如,高迁移率族蛋白B1(High Mobility Group Box 1,HMGB1)可在巨噬细胞中发生p300依赖的乳酸化,促进其胞质转位及外泌体释放,进而破坏内皮屏障功能、增加血管通透性[9-10]。转录因子YY1和HIF-1α的乳酸化分别调控血管生成和肿瘤微环境相关基因表达。代谢酶如ALDOA和α-烯醇酶的乳酸化修饰,则可反馈调节糖酵解活性,影响细胞代谢稳态[11]。
3 乳酸化修饰在脓毒症器官损伤中的作用 3.1 肺脏损伤肺脏常为脓毒症中最先受累的器官[12]。其损伤机制除缺氧所致的无氧代谢增强外,还包括细胞因子直接作用及肺内细胞葡萄糖代谢加速[13]。乳酸积累可促进巨噬细胞中HMGB1的乳酸化及外泌体释放,后者作为损伤相关分子模式(damage-associated molecular patterns, DAMP)分子,通过TGF-β信号通路破坏上皮屏障功能,下调紧密连接蛋白表达,加剧炎症及肺组织损伤[14]。乳酸还可打破M1/M2巨噬细胞功能平衡,加重肺损伤[15]。在肺纤维化过程中,乳酸通过增强促纤维化基因启动子区的组蛋白乳酸化,驱动成纤维细胞向肌成纤维细胞转化,促进胶原与细胞外基质过度沉积,最终导致肺纤维化,抑制p300可降低乳酸化水平并延缓纤维化进展[16-17]。
3.2 心脏损伤乳酸化修饰在脓毒症相关心肌损伤中具有双重作用。一方面,乳酸可通过促进Snail1蛋白的乳酸化及其核转位,激活TGF-β/Smad2通路,诱导内皮–间质转化(endothelial-to-mesenchymal transition,EndoMT),加剧心脏纤维化[18-21]。另一方面,在心肌缺血区域,乳酸化能稳定HIF-1α,促进血管内皮生长因子等表达,刺激血管新生,改善心肌灌注[22-23]。这表明乳酸化在心脏修复与损伤中具语境依赖性。
3.3 肝脏损伤乳酸在脓毒症相关肝损伤中通过诱导组蛋白H3K18乳酸化,富集于肝星状细胞(Hepatic Stellate Cell, HSC)激活相关基因启动子区,促进SOX9过表达,进而激活NF-κB通路,引发炎症细胞因子大量释放,损伤肝细胞并激活HSC[24-26]。活化的HSC分泌细胞外基质(Extracellular Matrix,ECM),在狄氏间隙中积累,导致肝窦毛细血管化及肝纤维化[27-28]。
3.4 肾脏损伤脓毒症相关急性肾损伤(Sepsis-Induced Acute Kidney Injury, SAKI)中,SIRT3下调导致丙酮酸脱氢酶A1乙酰化失活,乳酸积聚;过量乳酸诱导线粒体分裂蛋白Fis1发生K20位点乳酸化,引发线粒体凋亡,加重肾小管细胞损伤[29-30]。同时,乳酸通过维持mTOR活化抑制自噬起始,导致受损细胞器与蛋白堆积,加剧炎症与细胞死亡[31-33]。此外,乳酸化HMGB1可促进中性粒细胞胞外陷阱(Neutrophil Extracellular Traps,NETs)释放促进AKI发生[34]。乳酸能激活MAPK、PI3K-Akt及TGF-β等信号通路,推动肾成纤维细胞活化与肾脏纤维化[35-37]。
3.5 脑损伤脓毒症相关脑损伤中,乳酸化修饰呈现复杂双面性:一方面可促进抗炎基因如Arg1转录,诱导M2型巨噬细胞极化,抑制神经炎症[38];另一方面,高乳酸环境通过p300催化HMGB1乳酸化,促进其释放入血,破坏血脑屏障,介导全身性炎症反应向中枢蔓延,加剧脑损伤[39]。
3.6 乳酸化驱动的多器官串扰脓毒症中多器官损伤并非孤立事件,而是由代谢–免疫–炎症轴联动驱动的系统性过程。乳酸化修饰在其中起到核心桥梁作用,介导器官间恶性串扰。某一器官释放的乳酸化蛋白(如HMGB1)可通过循环系统激活远处器官的炎症与内皮损伤,形成跨器官“炎症风暴”。同时,肝功能衰竭加剧全身代谢紊乱,进一步强化糖酵解—乳酸—乳酸化正反馈环路,最终导致多器官功能障碍综合征(MODS)[40-41]。
4 结论与展望乳酸作为糖酵解关键代谢物,在脓毒症中不仅是能量底物,更是重要的免疫代谢信号分子,通过乳酸化修饰广泛参与脓毒症多器官损伤的调控。近年来研究提示乳酸化相关基因(如S100A11、CCNA2)在脓毒症诊断与预后评估中具潜在价值[42],为实现脓毒症精准诊疗提供新方向。
未来研究需整合多组学数据与人工智能算法,构建乳酸化修饰与基因组、转录组、蛋白组及临床表型的综合分析模型,以期筛选出可靠生物标志物与治疗靶点。在治疗策略上,针对乳酸化通路的干预(如LDH-A抑制剂、糖酵解调控剂)可能通过抑制过度炎症、阻断器官间恶性串扰,改善脓毒症预后。然而,其临床转化仍面临技术、安全性与有效性等多重挑战,需依托跨学科合作,结合计算生物学与严格临床试验,共同推动脓毒症精准医学的发展。
利益冲突 所有作者声明无利益冲突
| [1] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [2] | 李佳, 周人杰. 急诊重症感染的诊治策略及进展[J]. 中华急诊医学杂志, 2024, 33(6): 745-748. DOI:10.3760/cma.j.issn.1671-0282.2024.06.003 |
| [3] | Alharbi AS, Sanyi RH, Azhar EI. Bacteria and host: what does this mean for sepsis bottleneck?[J]. World J Emerg Med, 2025, 16(1): 10-17. DOI:10.5847/wjem.j.1920-8642.2025.001 |
| [4] | Chen LH, Huang LX, Gu Y, et al. Lactate-lactylation hands between metabolic reprogramming and immunosuppression[J]. Int J Mol Sci, 2022, 23(19): 11943. DOI:10.3390/ijms231911943 |
| [5] | Ishihara S, Hata KJ, Hirose K, et al. The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer[J]. Sci Rep, 2022, 12(1): 6261. DOI:10.1038/s41598-022-10143-w |
| [6] | Yang LB, Gilbertsen A, Xia H, et al. Hypoxia enhances IPF mesenchymal progenitor cell fibrogenicity via the lactate/GPR81/HIF1α pathway[J]. JCI Insight, 2023, 8(4): e163820. DOI:10.1172/jci.insight.163820 |
| [7] | Xie YM, Hu HX, Liu MT, et al. The role and mechanism of histone lactylation in health and diseases[J]. Front Genet, 2022, 13: 949252. DOI:10.3389/fgene.2022.949252 |
| [8] | Hu Y, He ZL, Li ZJ, et al. Lactylation: the novel histone modification influence on gene expression, protein function, and disease[J]. Clin Epigenetics, 2024, 16(1): 72. DOI:10.1186/s13148-024-01682-2 |
| [9] | Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection[J]. Annu Rev Immunol, 2011, 29: 139-162. DOI:10.1146/annurev-immunol-030409-101323 |
| [10] | Yang K, Fan M, Wang XH, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis[J]. Cell Death Differ, 2022, 29(1): 133-146. DOI:10.1038/s41418-021-00841-9 |
| [11] | Wang JY, Wang ZY, Wang QX, et al. Ubiquitous protein lactylation in health and diseases[J]. Cell Mol Biol Lett, 2024, 29(1): 23. DOI:10.1186/s11658-024-00541-5 |
| [12] | Palsson-McDermott EM, O'Neill LAJ. The Warburg effect then and now: from cancer to inflammatory diseases[J]. Bioessays, 2013, 35(11): 965-973. DOI:10.1002/bies.201300084 |
| [13] | Iscra F, Gullo A, Biolo G. Bench-to-bedside review: lactate and the lung[J]. Crit Care, 2002, 6(4): 327-329. DOI:10.1186/cc1519 |
| [14] | Kodera Y, Kohno T, Konno T, et al. HMGB1 enhances epithelial permeability via p63/TGF-β signaling in lung and terminal bronchial epithelial cells[J]. Tissue Barriers, 2020, 8(4): 1805997. DOI:10.1080/21688370.2020.1805997 |
| [15] | Wei YG, Guo H, Chen SX, et al. Regulation of macrophage activation by lactylation in lung disease[J]. Front Immunol, 2024, 15: 1427739. DOI:10.3389/fimmu.2024.1427739 |
| [16] | Cui HC, Xie N, Banerjee S, et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation[J]. Am J Respir Cell Mol Biol, 2021, 64(1): 115-125. DOI:10.1165/rcmb.2020-0360OC |
| [17] | 曹成龙, 马向丽, 刘贻晶, 等. 乳酸化修饰在脓毒症作用机制中的研究进展[J]. 中华急诊医学杂志, 2024, 33(4): 584-590. DOI:10.3760/cma.j.issn.1671-0282.2024.04.023 |
| [18] | Lai CC, Lee MG, Lee WC, et al. Susceptible period for cardiovascular complications in patients recovering from sepsis[J]. CMAJ, 2018, 190(36): E106-E1069. DOI:10.1503/cmaj.171284 |
| [19] | Abudurexiti S, Xu SH, Sun ZP, et al. Glucose metabolic reprogramming-related parameters for the prediction of 28-day neurological prognosis and all-cause mortality in patients after cardiac arrest: a prospective single-center observational study[J]. World J Emerg Med, 2024, 15(3): 197-205. DOI:10.5847/wjem.j.1920-8642.2024.047 |
| [20] | Chen ZT, Gao QY, Wu MX, et al. Glycolysis inhibition alleviates cardiac fibrosis after myocardial infarction by suppressing cardiac fibroblast activation[J]. Front Cardiovasc Med, 2021, 8: 701745. DOI:10.3389/fcvm.2021.701745 |
| [21] | Fan M, Yang K, Wang XH, et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction[J]. Sci Adv, 2023, 9(5): eadc9465. DOI:10.1126/sciadv.adc9465 |
| [22] | Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management[J]. J Intensive Care, 2016, 4: 22. DOI:10.1186/s40560-016-0148-1 |
| [23] | Zhu WG, Guo SY, Sun JY, et al. Lactate and lactylation in cardiovascular diseases: current progress and future perspectives[J]. Metabolism, 2024, 158: 155957. DOI:10.1016/j.metabol.2024.155957 |
| [24] | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(7): 397-411. DOI:10.1038/nrgastro.2017.38 |
| [25] | Rho H, Terry AR, Chronis C, et al. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis[J]. Cell Metab, 2023, 35(8): 1406-1423. DOI:10.1016/j.cmet.2023.06.013 |
| [26] | Trogisch FA, Abouissa A, Keles M, et al. Endothelial cells drive organ fibrosis in mice by inducing expression of the transcription factor SOX9[J]. Sci Transl Med, 2024, 16(736): eabq4581. DOI:10.1126/scitranslmed.abq4581 |
| [27] | Fan XD, Zheng HB, Fan XS, et al. Increase of SOX9 promotes hepatic ischemia/reperfusion (IR) injury by activating TGF-β1[J]. Biochem Biophys Res Commun, 2018, 503(1): 215-221. DOI:10.1016/j.bbrc.2018.06.005 |
| [28] | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis[J]. Adv Drug Deliv Rev, 2017, 121: 27-42. DOI:10.1016/j.addr.2017.05.007 |
| [29] | Wu ZX, Liu WQ, Tang L, et al. Lactate-mitochondrial crosstalk: a new direction in the treatment of sepsis-induced acute kidney injury[J]. Cell Biol Int, 2024, 48(11): 1621-1624. DOI:10.1002/cbin.12240 |
| [30] | An S, Yao Y, Hu HB, et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation[J]. Cell Death Dis, 2023, 14(7): 457. DOI:10.1038/s41419-023-05952-4 |
| [31] | Li T, Zhao J, Miao SY, et al. Dynamic expression and roles of sequestome-1/p62 in LPS-induced acute kidney injury in mice[J]. Mol Med Rep, 2018, 17(6): 7618-7626. DOI:10.3892/mmr.2018.8809 |
| [32] | Tan CY, Gu J, Li T, et al. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy[J]. Int J Mol Med, 2021, 47(3): 19. DOI:10.3892/ijmm.2021.4852 |
| [33] | Kimura T, Isaka Y, Yoshimori T. Autophagy and kidney inflammation[J]. Autophagy, 2017, 13(6): 997-1003. DOI:10.1080/15548627.2017.1309485 |
| [34] | Zhu L, Zheng Q, Liu XD, et al. HMGB1 lactylation drives neutrophil extracellular trap formation in lactate-induced acute kidney injury[J]. Front Immunol, 2025, 15: 1475543. DOI:10.3389/fimmu.2024.1475543 |
| [35] | Qiao J, Tan Y, Liu HC, et al. Histone H3K18 and ezrin lactylation promote renal dysfunction in sepsis-associated acute kidney injury[J]. Adv Sci (Weinh), 2024, 11(28): e2307216. DOI:10.1002/advs.202307216 |
| [36] | Wang YT, Li HY, Jiang SM, et al. The glycolytic enzyme PFKFB3 drives kidney fibrosis through promoting histone lactylation-mediated NF-κB family activation[J]. Kidney Int, 2024, 106(2): 226-240. DOI:10.1016/j.kint.2024.04.016 |
| [37] | Sabra RT, Bekhit AA, Sabra NT, et al. Nebivolol ameliorates sepsis-evoked kidney dysfunction by targeting oxidative stress and TGF-β/Smad/p53 pathway[J]. Sci Rep, 2024, 14(1): 14735. DOI:10.1038/s41598-024-64577-5 |
| [38] | Li RB, Yang Y, Wang HY, et al. Lactate and lactylation in the brain: current progress and perspectives[J]. Cell Mol Neurobiol, 2023, 43(6): 2541-2555. DOI:10.1007/s10571-023-01335-7 |
| [39] | Liu JY, Zhao FY, Qu Y. Lactylation: a novel post-translational modification with clinical implications in CNS diseases[J]. Biomolecules, 2024, 14(9): 1175. DOI:10.3390/biom14091175 |
| [40] | Borges A, Bento L. Organ crosstalk and dysfunction in sepsis[J]. Ann Intensive Care, 2024, 14(1): 147. DOI:10.1186/s13613-024-01377-0 |
| [41] | Zhang J, Wu D, Zeng F, et al. Lactate metabolic reprogramming and histone lactylation modification in sepsis[J]. Int J Biol Sci, 2025, 21(11): 5034-5055. DOI:10.7150/ijbs.116088 |
| [42] | Li SL, Shen YZ, Wang CL, et al. Exploring the prognostic and diagnostic value of lactylation-related genes in sepsis[J]. Sci Rep, 2024, 14(1): 23130. DOI:10.1038/s41598-024-74040-0 |
 2025, Vol. 34
2025, Vol. 34