高脂血症会导致或加重重症急性胰腺炎(severe acute pancreatitis,SAP),这一观点已得到广泛认可[1-2]。众所周知,SAP常常会引发胰外脏器的损伤,急性胰腺炎肺损伤(acute pancreatitis associated-lung injury,APALI)是SAP最常见的并发症。针对伴随高脂血症SAP肺损伤的研究,目前国内外的相关研究较少。罗格列酮(rosiglitazone,ROSI)属于过氧化物酶体增殖物激活受体-γ (peroxisome proliferator- activated receptors-γ,PPAR-γ)激动剂,能够调节脂质代谢、脂肪细胞分化和胰岛素敏感性,临床上主要用于2型糖尿病的治疗。近年来PPAR-γ激动剂在炎症调控中的作用引起人们的广泛关注[3-5]。本研究观察ROSI对伴随高脂血症SAP大鼠肺损伤的影响,探讨其对高脂血症合并SAP时肺损伤的保护作用及其机制。
1 材料与方法 1.1 实验动物与分组SPF级雄性SD大鼠120只、体质量150~200 g(湖北省疾病预防控制中心提供)。给予自制的脂肪乳剂(30%猪油+10%胆固醇+20%吐温+5%胆盐+1%丙基硫样嘧啶,由武汉大学人民医院动物实验中心协助配制)每天灌胃一次,灌胃量(1 mL/100 g),连续灌胃两周,其余时间给予普通饲料喂养。两周后随机分为6组:高脂血症组(HL,n=20)、高脂血症合并SAP组(HP,n=20)、罗格列酮干预组(HRP,n=20)、罗格列酮拮抗组(HRGP,n=20)、罗格列酮对照组(HR,n=20)、拮抗剂对照组(HG,n=20)。
1.2 动物模型制备及处理实验前大鼠禁食12 h,自由饮水。10%水合氯醛腹腔注射(0.3 mL/100 g)麻醉,无菌操作下行上腹正中切口进腹,采用1 mL注射器针头穿过十二指肠对系膜缘经乳头逆行插入主胰管,以约0.1 mL/min恒速向主胰管注射5%牛磺胆酸钠溶液(1 mL/kg,Sigma公司)制备SAP模型。HP组、HRP组及HRGP组逆行胰胆管注射5%牛磺胆酸钠建立SAP模型。HL组、HR组和HG组操作同HP组、HRP组及HRGP组,但胰胆管内仅注入等容量生理盐水;HRP组和HR组于造模前1 h经右侧股静脉注射ROSI (6 mg/kg);HRGP组于注射ROSI前30 min经左侧股静脉注射GW9662(GW9662为特异性PPAR-γ激动剂的拮抗剂,0.3 mg/kg,Enzo Life Sciences公司),其余操作同HRP组;HG组仅于造模前30 min经左侧股静脉注射GW9662(0.3 mg/kg)。
1.3 标本采集术后12 h分批剖杀大鼠。心脏采血,离心后分离血清-20 ℃保存备用;取部分胰腺组织和肺组织4%多聚甲醛固定用于光镜组织病理学检查及NF-κB p65蛋白免疫组织化学检测;其余肺组织立即经液氮冻存后转入-80 ℃冰箱保存,用于检测肺组织TNF-α、ICAM-1蛋白表达情况。
1.4 指标检测方法胰腺、肺组织病理学检查:胰腺、肺组织4%多聚甲醛固定、石蜡包埋制片,HE染色后光镜下观察,以schmidt法[6]对胰腺损伤的程度进行病理学评分,参照文献[7]对肺组织行病理学评分。
血清淀粉酶(AMY)、甘油三酯(TG)、总胆固醇(TC)在武汉大学人民医院检验中心予以检测。
肺组织NF-κB p65蛋白表达检测:大鼠肺组织用10%甲醛固定24 h,石蜡包埋切片,采用SP法行免疫组织化学检查。NF-κB p65蛋白定位于细胞质或胞核染色呈棕黄色为阳性。
Western-Blot检测肺组织ICAM-1和TNF-α蛋白的表达:冻存的各组肺组织以蛋白裂解液匀浆,冰上孵育30 min后,4 ℃、13 000 r/min离心30 min,取上清液,得总蛋白;BCA法测定蛋白浓度;取40 μg蛋白样品上样行SDS-PAGE凝胶电泳,蛋白电转至NC膜,10%脱脂奶粉封闭2 h,加入ICAM-1兔抗大鼠单克隆抗体(1∶ 800,SantaCruz公司)、TNF-α兔抗大鼠单克隆抗体(1∶ 1 000,abcam公司)或β-actin一抗(1∶ 1 000,cell signaling technology公司),置于4℃冰箱振荡孵育过夜后,TBST漂洗,加辣根过氧化酶标记二抗(1∶ 3 000),室温孵育1 h后以ECL化学发光试剂于暗室显影,凝胶图像分析系统检测蛋白质印迹条带。
1.5 统计学方法各组数据以均数±标准差(x±s)表示,多组间比较采用单因素方差分析ANOVA 检验,组间两两比较用LSD-t检验,方差不齐则进行秩和检验,SPSS 17.0统计软件进行分析,以P<0.05为差异有统计学意义。
2 结果 2.1 血清学指标血清AMY、TG、TC和肺W/D的变化:HL组和HP组的TG、TC的血清水平均明显高于HR组和HRP组(均P<0.05);HRP组的各项指标均较HP和HRGP组有所降低(均P<0.05 ),但是HP组各项指标均与HRGP组差异无统计学意义;HR组除血清TG、TC明显低于HL组和HG组外(均P<0.05),其余指标在HL、HR、HG三组之间差异无统计学意义(表 1)。
| 组别 | AMY(U/L) | TG(mmol/L) | TC(mmol/L) | 肺W/D |
| HL组 | 1 139.3±35.6 | 1.24±0.28 | 14.86±1.47 | 1.81±0.13 |
| HP组 | 6 501.9±3770.0a | 1.14±0.08 | 12.42±0.96 | 3.14±0.16a |
| HRP组 | 2 006.9±331.9ab | 0.58±0.12ab | 7.36±0.95ab | 2.17±0.35ab |
| HRGP组 | 5 922.2±925.9ac | 1.18±0.12c | 10.08±1.048ac | 3.06±0.12ac |
| HR组 | 1 070.8±67.0bcd | 0.41±0.17abd | 6.52±2.04abd | 1.76±0.23bcd |
| HG组 | 1 012.4±94.7bcd | 1.20±0.04ce | 11.41±1.39ce | 1.83±0.18bcd |
| 注:与HL 组比较,a P<0.05;与HP组比较,bP<0.05;与 HRP组比较,cP<0.05;与 HRGP 组比较,dP<0.05;与 HR 组比较,eP<0.05 | ||||
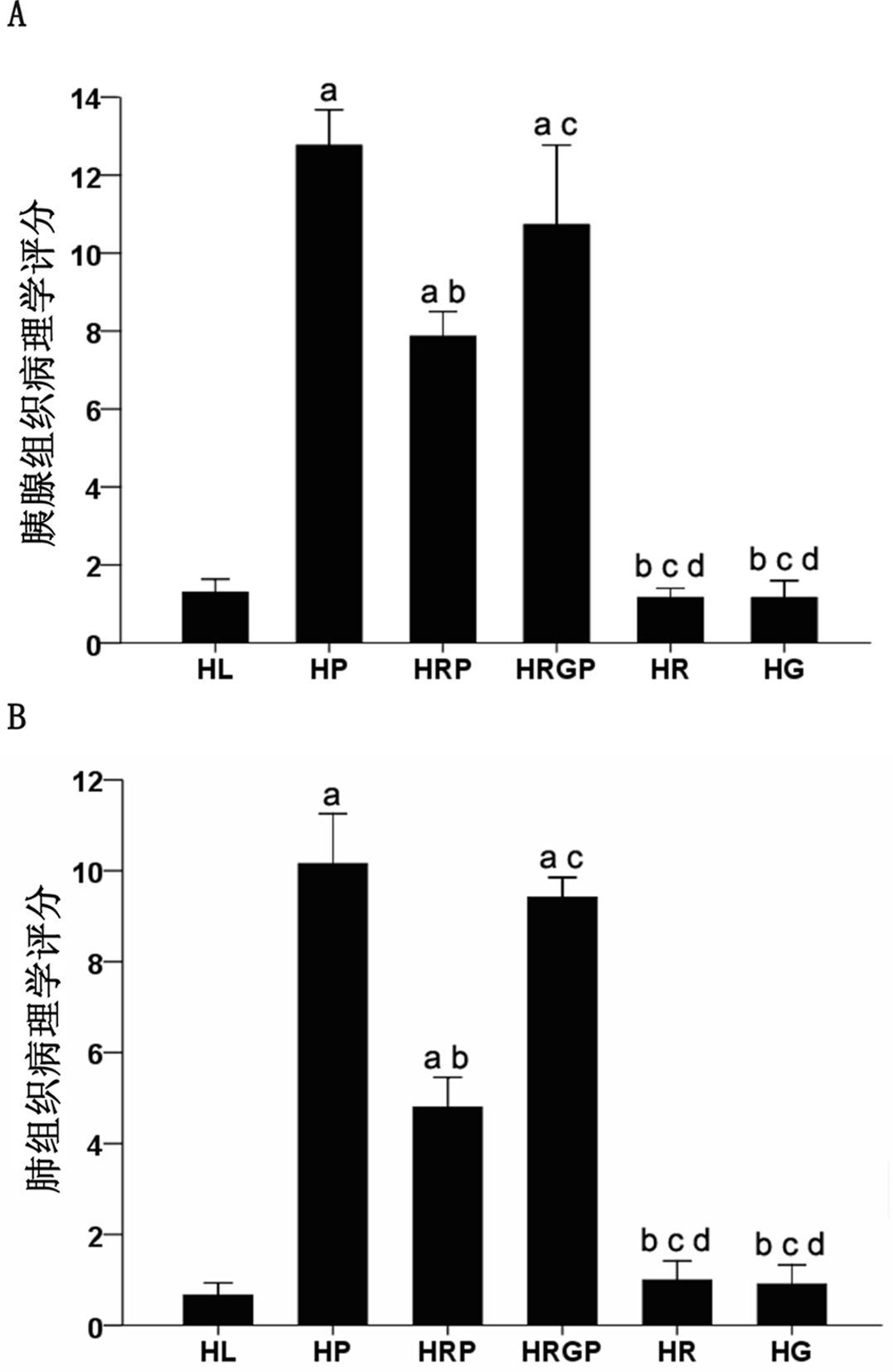
HL组大鼠胰腺腺泡没有异常但胰腺间质及胰周组织脂肪明显增生。HP组胰腺结构被破坏,大片凝固坏死,腺泡结构消失,残存胰腺小叶孤立丁坏死区,伴出血及大量炎症细胞浸润;HRP组胰腺出血、坏死范围缩小,炎性细胞浸润减轻;而HRGP组大鼠胰腺出血、炎性细胞浸润及坏死情况与HP组相似,较HRP组明显加重;HR组和HG组胰腺组织结构基本正常,胰腺间质及胰周组织可见少量脂肪组织增生。各组胰腺组织病理评分见图 2。
|
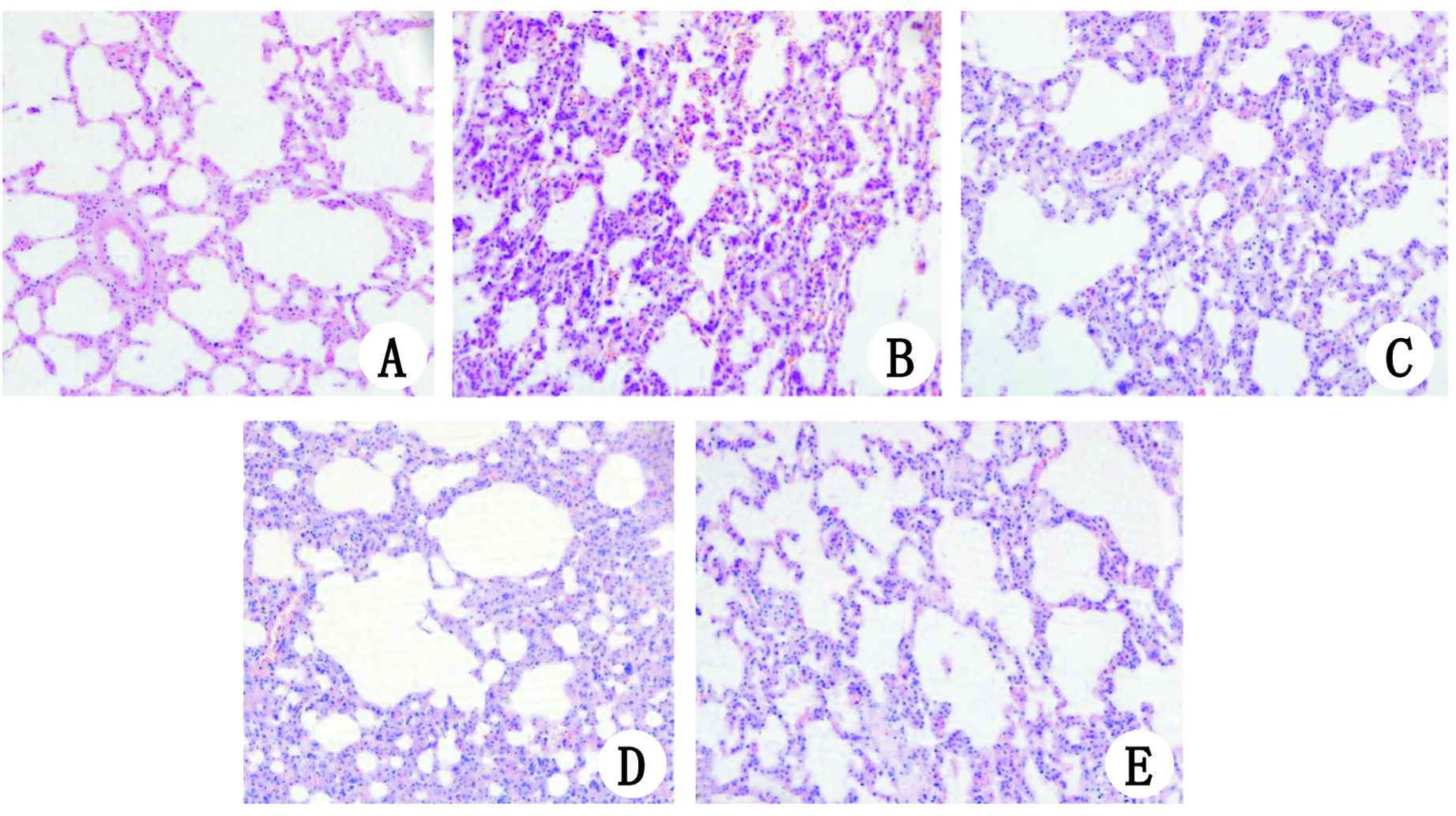
| A、E、F分别为HL组、HR组和HG组,肺脏组织结构未见明显异常;B、D分别为HP组、HRGP组,肺组织间质明显充血、水肿,大量炎症细胞浸润;C为HRP组,可见肺脏间质充血、水肿减轻,炎症细胞浸润减少 图 1 各组肺组织病理学改变 HE染色(×200) Figure 1 Morphological changes of the lung tissue in all groups (×200) |
|
|
|
| 与HL组比较,aP<0.05;与HP 组比较,bP<0.05;与HRP组比较,cP<0.05;与HRGP组比较,dP<0.05 图 2 各组大鼠胰腺、肺组织的病理评分 Figure 2 Comparison of the total pathological score in the pancreas(A) and lung(B) among groups |
|
|
HL组肺脏结构基本正常;HP组和HRGP组肺组织间质明显水肿,大量炎症细胞浸润,病理评分较HL组明显升高(均P<0.05),而HP组和HRGP组两者之间比较,差异无统计学意义(P>0.05);HRP组肺脏间质水肿减轻,炎症细胞浸润减少,肺组织病理评分较HP组和HRGP组明显降低,差异有统计学意义(均P<0.05);HR组和HG组肺脏结构基本正常,与HL组比较,差异无统计学意义(P>0.05)(图 1)。各组肺脏组织病理评分见图 2。
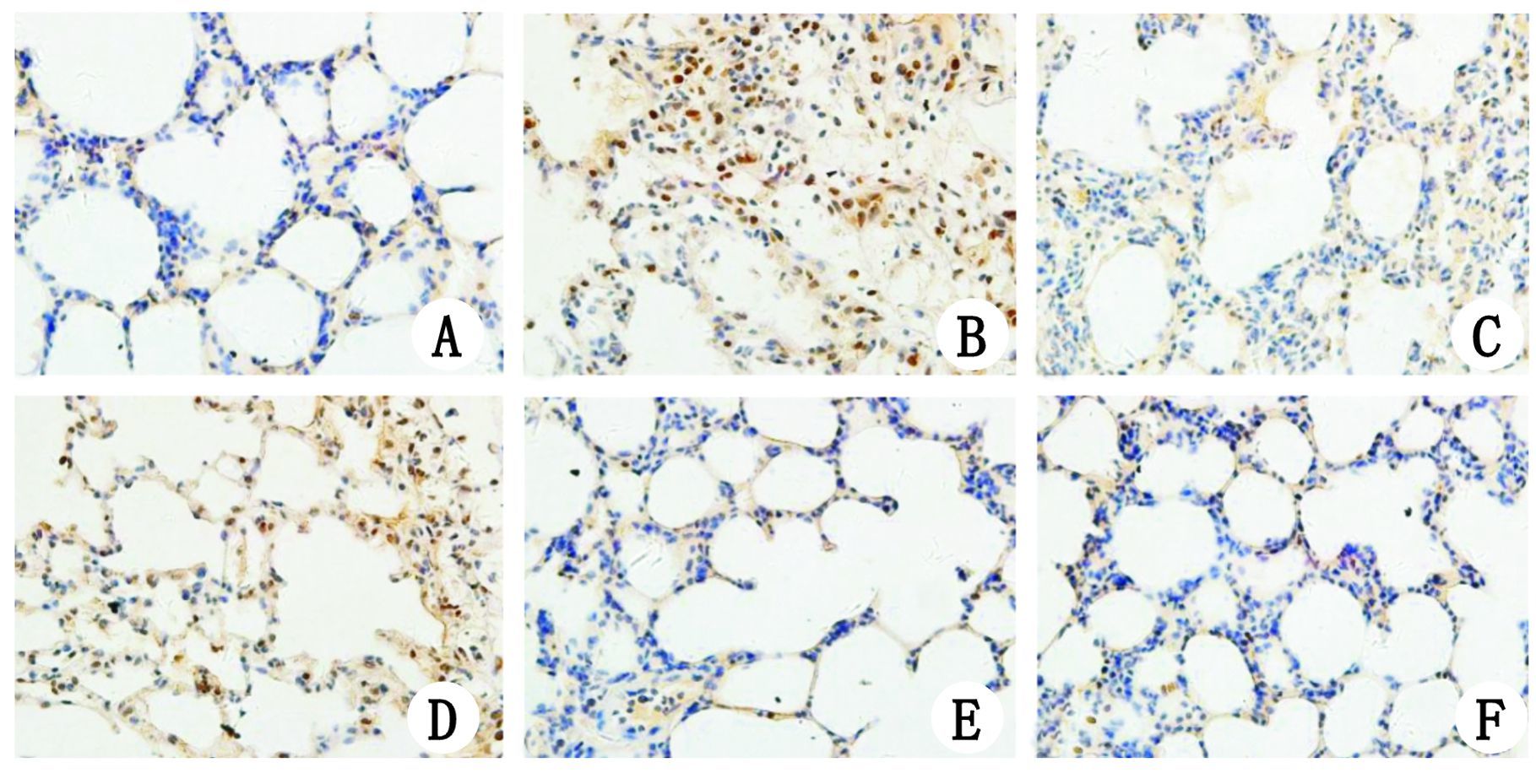
2.4 各组肺组织NF-κB p65表达免疫组化结果NF-κB p65阳性表达为视野内组织被染成棕黄色。HL组、HR组、HG组肺组织中仅可见胞浆内极微量表达,HP组及HRGP组胞核内大量表达,HRP组较HP组及HRGP组胞核内表达明显减少(图 3)。
|
| A:HL组大鼠肺组织仅可见胞浆内微量表达,细胞核内未见表达NF-κB p65;B,D:分别为HP组和HRGP组,均可见胞核内大量表达NF-κB p65;C:HRP组胞核内NF-κB p65表达明显较HP组和HRGP组减少,胞浆内仅微量表达;E,F:分别为HR组和HG组,可见胞浆内极微量表达NF-κB p65,细胞核内未见表达 图 3 各组肺组织NF-κB p65表达免疫组化结果(×400) Figure 3 Immunohistochemical localisation of NF-κB p65 in the lung tissue of all groups (×400) |
|
|
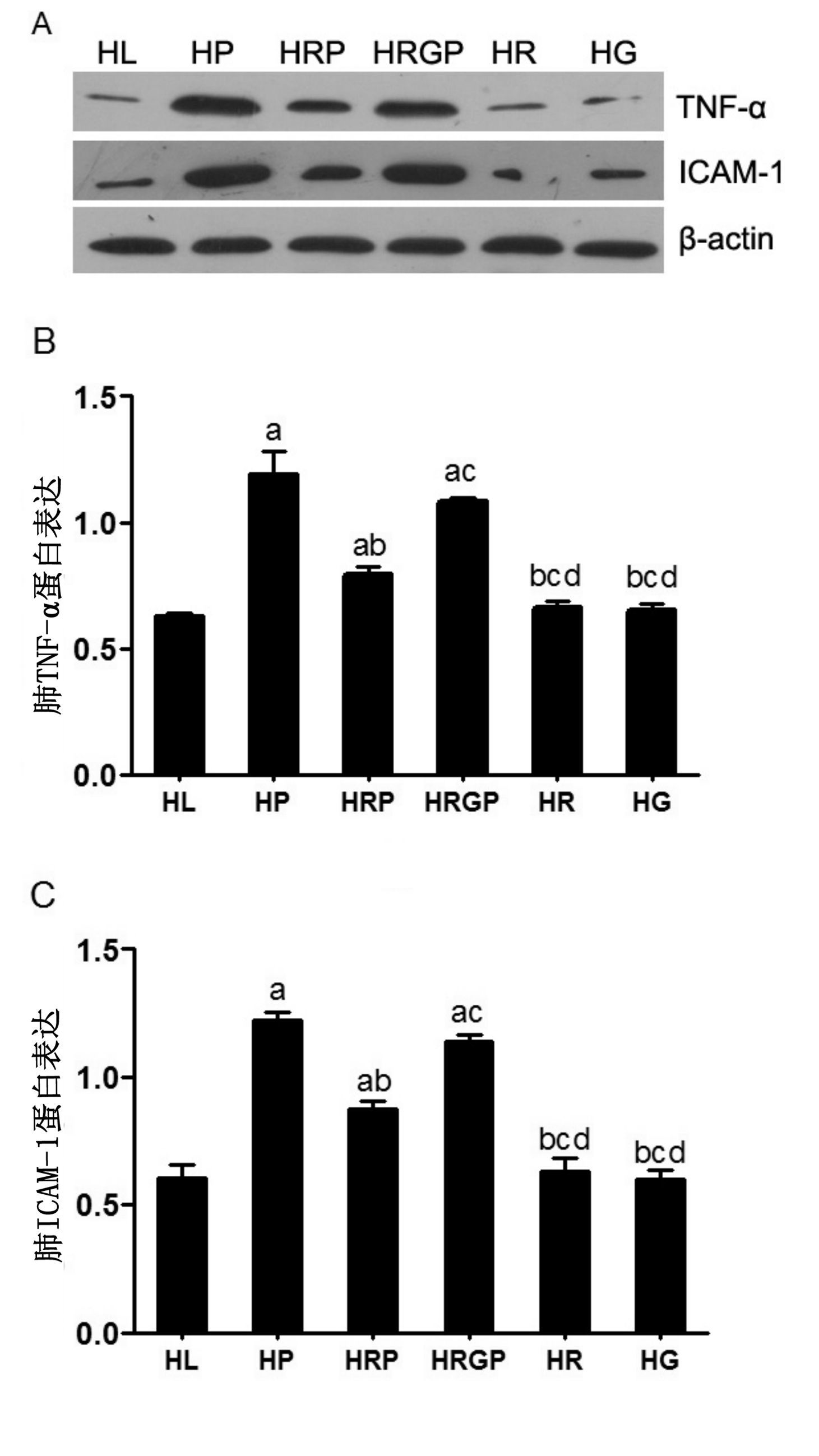
HP组和HRGP组大鼠肺组织TNF-α和ICAM-1的蛋白表达水平均明显高于HL组、HR组及HG组(均P<0.05);HRP组TNF-α和ICAM-1的蛋白表达水平均明显低于HP组及HRGP组(均P<0.05);而HL组、HR组及HG组大鼠肺组织TNF-α和ICAM-1蛋白表达水平之间差异无统计学意义(均P>0.05)(图 4)。
|
| 与HL组比较,aP<0.05;与HP组比较,bP<0.05;与HRP组比较,cP<0.05;与 HRGP组比较,dP<0.05 图 4 各组大鼠肺组织TNF-α和ICAM-1蛋白表达情况 Figure 4 Western blotting used to measure the activation of TNF-α and ICAM-1 in lung tissue of all groups |
|
|
罗格列酮(ROSI)属于人工合成高选择性PPAR-γ的激动剂,能够调节脂质代谢、脂肪细胞分化和增强胰岛素敏感性,减轻胰岛素抵抗,临床上主要用于治疗2型糖尿病。目前已有研究证明急性胰腺炎模型中ROSI可通过PPAR-γ途径发挥抗炎作用[8],针对ROSI的抗炎和调节脂质代谢两大功能,其能否对伴高脂血症SAP大鼠肺损伤产生其相应的作用,尚未见相关报道。
有研究表明,高脂血症能加重胰腺炎及其胰外脏器的损伤[9]。在高脂条件下,胰内和胰周高浓度的甘油三酯被胰脂酶水解造成局部大量脂肪酸聚集,大量脂肪酸和胰腺毛细血管释放的溶血卵磷脂超过了白蛋白所能结合的数量,而使胰腺细胞膜溶化和毛细血管上皮造成损害,从而激发或加重了SAP[10-11]。另一原因可能是在伴有高脂的状态下,机体脂质过氧化物含量升高,超氧化物歧化酶及过氧化物酶活性降低,机体氧自由基生成增多、清除减少,因而炎症反应较单纯的SAP组更为严重。肺脏是SAP时最先累及的胰外器官,同样的原因,高脂血症也能够加重SAP肺损伤的程度。本实验HRP组在造模前1 h经股静脉注射ROSI预处理后,血清TC、TG、AMY明显较HP组降低。而HRGP组大鼠在给予ROSI前给予GW9662(PPAR-γ拮抗剂)后,其血清TC、TG、AMY指标则没有明显改变,与HP组相似,以上结果均可说明ROSI能够显著降低血脂水平,从而减轻高脂血症对SAP时肺损害的程度。
研究表明,在SAP模型中,肺组织中活性氧、脂质过氧化物迅速增加,同时随着IκBα的降解,NF-κB被激活,TNF-α、IL-1、IL-6等细胞因子、黏附分子(ICAM-1、VCAM-1等)的基因表达明显上调,这些炎症因子与NF-κB p65亚单位核易位有关,并将进一步导致中性粒细胞的黏附和浸润增加,中性粒细胞被激活后,活性氧产生明显增加,可进一步激活NF-κB,引起白细胞贴壁、黏附,导致胰腺及胰外脏器炎性细胞浸润,从而导致胰外脏器的损伤进一步加重[12-13]。而通过减少AP动物模型中NF-κB的激活,胰腺炎及胰外脏器的损伤严重程度能够显著降低[14-15]。本实验通过免疫组织化学检测肺脏组织中NF-κB p65的表达变化,发现NF-κB p65在HP组的肺组织细胞核内表达明显增强,并定位于细胞核内发挥基因转录调节作用。而HRP组胰腺组织NF-κB p65的表达较HP组明显下调,NF-κB p65的活化受到明显抑制。而在给予ROSI前给予GW9662,其能够特异性的抑制PPAR-γ激动剂的作用,即HRGP组,其肺组织NF-κB p65的表达则明显升高,这也从另一方面证明ROSI能够有效抑制NF-κB的活化。
NF-κB活化后能调控一系列基因的表达,具有这些启动子/增强子的基因有IL-1、IL-6、TNF-α、iNOS、ICAM-1等。在SAP的发病过程中,这些物质均能够刺激炎性细胞向内皮细胞表而黏附、聚集、渗出而发挥作用。TNF-α是SAP最早升高的细胞因子,可直接作用于血管内皮细胞,导致组织出血坏死。还可作为重要的始发因素作用于多种细胞,促进其他细胞因子的产生,引起连锁反应[16]。有研究表明炎症过程中ICAM-1的表达受TNF-α的调控[17]。ICAM-l可与粒细胞表面的整合素相互作用,通过介导白细胞的紧密粘附与游出,从而导致局部和远处器官病损[18]。本实验中HP组肺组织TNF-α、ICAM-l蛋白表达量明显高于HL组,而预先给予ROSI处理后,可以显著减少肺组织TNF-α和ICAM-1 蛋白的表达。而给予ROSI前给予GW9662,则TNF-α和ICAM-1 蛋白的表达量明显升高,与HP组表达量相似。因此笔者认为ROSI可能是通过抑制NF-κB的活化,减少TNF-α和ICAM-1表达而起到减轻高脂血症合并SAP时肺损伤的作用。
本研究通过血清指标、病理评分及蛋白质水平分析PPAR-γ激动剂ROSI对伴随高脂血症SAP大鼠肺损伤的保护作用,证明其保护机制可能与降低血脂水平和抑制NF-κB这一转录因子的激活有关,而NF-κB激活的减少,使得其下游的炎性因子及黏附分子表达相应减少。TNF-α、ICAM-1等炎症介质的下调,使中性粒细胞黏附、聚集、渗出减少,从而减轻了肺组织的损伤。但是PPAR-γ激动剂对伴高脂血症SAP时肺损伤更详细的作用机制仍有待进一步探讨。
| [1] | Blom DJ, Byrnes P, Jones S, et al. Dysbetalipoproteinaemia-clinical and pathophysiological features[J]. South Afr Med J , 2002, 92 (11) : 892-897 |
| [2] | Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis[J]. J Clin Gastroenterol , 2003, 36 (1) : 54-62 DOI:10.1097/00004836-200301000-00016 |
| [3] | Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation[J]. Eur J Pharmacol , 2004, 483 (1) : 79-93 DOI:10.1016/j.ejphar.2003.10.056 |
| [4] | Kim SK, Park CK, Lee SY, et al. Effects of rosiglitazone on the expression of PPAR-gamma and on the production of IL-6 and IL-8 in acute lung injury model using human pulmonary epithelial cells[J]. Trop J PharmRes , 2011, 10 (2) : 731-738 DOI:10.4314/tjpr.v10i6.5 |
| [5] | Lim HA, Lee EK, Kim JM, et al. PPAR gamma activation by baicalin suppresses NF-kappa B-mediated inflammation in aged rat kidney[J]. Biogerontology , 2012, 13 (2) : 133-145 DOI:10.1007/s10522-011-9361-4 |
| [6] | Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy[J]. Ann Surg , 1992, 215 (1) : 44-56 DOI:10.1097/00000658-199201000-00007 |
| [7] |
程石, 何三光, 张佳林. 肺泡巨噬细胞活化在急性坏死性胰腺炎大鼠肺损伤中的作用[J].
中华外科杂志 , 2002, 40 (8) : 609-612 Chen S, He SG, Zhang JL. The role of alveolar macrophage activation in rats with lung injury associated with acute necrotizing pancreatitis[J]. Chin J Surg , 2002, 40 (8) : 609-612 DOI:10.3760/j.issn.0529-5815.2002.08.015 |
| [8] | Ivashchenko CY, Duan SZ, Usher MG, et al. PPAR-gamma knockout in pancreatic epithelial cells abolishes the inhibitory effect of rosiglitazone on caerulein-induced acute pancreatitis[J]. Am J Physiol Gastrointest Liver Physiol , 2007, 293 (1) : 319-326 DOI:10.1152/ajpgi.00056.2007 |
| [9] | Czako L, Szabolcs A, Vajda A, et al. Hyperlipidemia induced by a cholesterol-rich diet aggravates necrotizing pancreatitis in rats[J]. Eur J Pharmacol , 2007, 572 (1) : 74-81 DOI:10.1016/j.ejphar.2007.05.064. |
| [10] | Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis[J]. World J Gastroenterol , 2008, 14 (28) : 4558-4561 DOI:10.3748/wjg.14.4558 |
| [11] | Ewald N, Hardt PD, Kloer HU. Severe hypertriglyceridemia and pancreatitis: presentation and management[J]. Curr Opin Lipidol , 2009, 20 (6) : 497-504 DOI:10.1097/MOL.0b013e3283319a1d |
| [12] | Zhang H, Cai CZ, Zhang XQ, et al. Breviscapine attenuates acute pancreatitis by inhibiting expression of PKC alpha and NF-kappa B in pancreas[J]. World J Gastroenterol , 2011, 17 (14) : 1825-1830 DOI:10.3748/wjg.v17.i14.1825 |
| [13] | Babu BI, Genovese T, Mazzon E, et al. Recombinant human activated protein C (Xigris) attenuates murine cerulein-induced acute pancreatitis via regulation of nuclear factor kappa B and apoptotic pathways[J]. Pancreas , 2012, 41 (4) : 619-628 DOI:10.1097/MPA.0b013e31823ca26d |
| [14] | Zhou M, Chen B, Sun H, et al. The protective effects of Lipoxin A(4) during the early phase of severe acute pancreatitis in rats[J]. Scand J Gastroenterol , 2011, 46 (2) : 211-219 DOI:10.3109/00365521.2010.525715 |
| [15] | Kim TH, Bae GS, Oh HJ, et al. 2' ,4' ,6' -Tris(methoxymethoxy) chalcone (TMMC) attenuates the severity of cerulein-induced acute pancreatitis and associated lung injury[J]. Am J Physiol Gastrointest Liver Physiol , 2011, 301 (4) : 694-706 DOI:10.1152/ajpgi.00210.2010 |
| [16] | Gulben K, Ozdemir H, Berberoglu U, et al. Melatonin modulates the severity of taurocholate-induced acute pancreatitis in the rat[J]. Dig Dis Sci , 2010, 55 (4) : 941-946 DOI:10.1007/s10620-009-0808-2 |
| [17] | Bernot D, Peiretti F, Canault M, et al. Upregulation of TNF-alpha-induced ICAM-1 surface expression by adenylate cyclase-dependent pathway in human endothelial cells[J]. J Cell Physiol , 2005, 202 (2) : 434-441 DOI:10.1002/jcp.20134. |
| [18] | Hsu WY, Chao YW, Tsai YL, et al. Resistin Induces Monocyte-Endothelial Cell Adhesion by Increasing ICAM-1 and VCAM-1 Expression in Endothelial Cells via p38MAPK-Dependent Pathway[J]. J Cell Physiol , 2011, 226 (8) : 2181-2188 DOI:10.1002/jcp.22555 |
 2016, Vol. 37
2016, Vol. 37




