2 消化系统疾病湖北省重点实验室,430060;
3 武汉大学人民医院中心实验室,430060
2 Key Laboratory of Hubei Province for Digestive System Disease, Wuhan 430060, Hubei, China;
3 Central Laboratory, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei, China
妊娠晚期合并急性胰腺炎(acute pancreatitis in late pregnancy,APILP)是一种极为凶险的急腹症,严重威胁母婴生命安全[1]。近年来,APILP发病率有升高趋势,主要与实验诊断技术的普及应用密切相关[2-3]。随着早期诊断和重症监护技术的进步,APILP后母婴的预后较前得到显著的改善,但是APILP相关的胎儿早产率高达47%[4],病死率高达3.6%[5-6]。
急性胰腺炎是一种全身性疾病,常伴有远隔脏器损伤,其中以肺损伤最为常见[7-8]。研究[2]表明APILP时胎儿呼吸窘迫综合征的发病率为4.45%,但是目前鲜有针对APILP相关胎儿肺脏损伤的机制研究报道。笔者前期研究发现,APILP时胎鼠肺脏存在显著的病理损伤,且随着病情进展逐渐加重[9]。如何早期预防和治疗APILP相关胎儿的肺脏损伤,对于改善母婴预后具有重要意义。p38MAPK是一种细胞内丝氨酸/苏氨酸蛋白激酶,可通过调控多种基因的转录与翻译过程,参与急胰腺炎时胰腺和远隔脏器的损伤过程[10-11],尤其是在急性胰腺炎相关性肺损伤中发挥重要作用[12]。本研旨在探讨抑制p38MAPK信号通路对APILP相关胎鼠肺脏损伤的保护作用及可能机制,以期为APILP相关胎儿肺脏损伤的治疗提供实验依据和干预靶点。
1 材料与方法 1.1 主要材料与试剂牛磺胆酸钠(STC)购自美国Sigma公司,p- p38MAPK、p38MAPK、NF-κB、HMGB1抗体购自美国CST公司,TNF-α、ICAM-1、MPO抗体购自英国Abcam公司,TNF-α酶联免疫吸附试验试剂盒购自武汉伊莱瑞特公司。
1.2 动物模型建立与实验分组SPF级SD妊娠大鼠24只(370~450 g,购自华中科技大学动物中心),采用随机数字法将其分为假手术(SO)组,妊娠晚期合并急性胰腺炎(APILP)组,p38MAPK抑制剂SB203580预处理(SB)组,每组8只大鼠。大鼠造模前禁食12 h,自由饮水。使用异氟烷(氧流量2 L/min,5%异氟烷诱导,2%~3%异氟烷维持果)麻醉后开腹,暴露大鼠胰腺和胆胰管。参考本团队前期研究[13-14],以0.1 mL/min恒速胰胆管逆行注射5% STC(0.06 mL/100 g体质量)建立急性胰腺炎模型。SB组大鼠在开腹建立APILP模型前0.5 h给予p38MAPK抑制剂SB203580(10 mg/kg体质量,溶解于5%DMSO)腹腔注射[11]。SO组开腹后翻动并暴露胰腺后即关腹。SO和APILP组大鼠在开腹前0.5 h给予对应体积的SB203580溶剂。各组大鼠术后均采用皮下注射补液(生理盐水,2 mL/100 g体质量)。
1.3 标本采集造模完成12 h后异氟烷诱导麻醉,剖腹取出胎鼠。采用真空采血管于妊娠大鼠下腔静脉采血,血液标本室温静置30 min后,2 500 r/min离心10 min。分离血清置于-80℃备用。快速获取大鼠胰头和部分胎鼠右肺,4%中性多聚甲醛固定,其余胎肺组织-80℃保存备用。
1.4 血清指标检测使用全自动生化仪(日本奥林巴斯)检测大鼠血清AMY水平,该检测由武汉大学人民医院检验科完成。根据试剂盒的说明,采用ELISA法检测大鼠血清中TNF-α水平。
1.5 妊娠大鼠胰腺及胎鼠肺脏组织病理学观察大鼠胰腺组织和胎鼠肺脏组织经4%中性多聚甲醛固定后,常规石蜡包埋、切片、苏木精 & 伊红(H & E)染色,光镜下观察。根据Schimidt等的方法对胰腺水肿、出血坏死、胰腺腺泡破坏、炎症及周围炎性浸润等方面进行病理评分,采用Bhatia等的方法对胎肺的病理损伤程度进行病理评分[15]。
1.6 免疫组织化学法检测胎肺组织NF-κB的表达胎肺组织的石蜡切片常规脱蜡、水化,置于枸橼酸钠盐溶液(10 mmol, pH 6.0)中高压(121℃,4 min)进行抗原修复,恢复室温后使用0.2% Triton ×100室温下通透45 min,PBS清洗3次后使用3%的H2O2去除内源性过氧化物酶,5%牛血清白蛋白封闭1h,滴加适当稀释的NF-κB抗体置于湿盒内4℃过夜。次日根据Eli Vision Super/HRP(福建迈新公司)免疫组织化学试剂盒说明书进行后续显影操作,并在光镜下观察分析,细胞内黄褐色染色即为NF-κB阳性表达。高倍镜下随机选择3个视野,统计每千个组织细胞中阳性染色的细胞数,进行统计学处理。
1.7 免疫荧光法检测胎肺组织内MPO的表达胎肺组织的石蜡切片的脱蜡、水化、抗原修复、通透同免疫组织化学检测。PBS清洗3次后采用10%驴血清封闭1 h,滴加适当稀释的MPO抗体置于湿盒内4℃过夜。次日洗脱切片上MPO抗体,使用驴抗兔荧光二抗室温下孵育1 h,应用含DAPI的抗荧光淬灭封片剂封片,荧光显微镜下观察分析,胞浆内有显著绿色荧光表达即为MPO阳性表达。高倍镜下随机选择3个视野,统计每千个组织细胞中MPO阳性表达的细胞数,进行统计学处理。
1.8 胎鼠肺脏组织内p-p38MAPK、p38MAPK、TNF-α、ICAM-1和HMGB1的表达水平检测使用RIPA匀浆裂解胎鼠肺脏组织提取总蛋白,BCA法检测蛋白浓度。使用12%SDS聚丙烯酰胺凝胶电泳分离蛋白质(30 μg蛋白/泳道),应用Bio-Rad转模系统激将蛋白转印至PVDF膜上,含10%脱脂奶粉TBST溶液封闭2 h。载有蛋白的PVDF膜经TBST清洗3次后,分别加入p-p38MAPK、p38MAPK、TNF-α、ICAM-1、HMGB1抗体稀释液,4℃下孵育过夜。次日加入适度稀释的荧光二抗,常温孵育1 h,TBST清洗3次后,采用奥德赛双色红外激光成像系统(美国LICOR公司)采集荧光信号并转化为灰度图,Quality One软件进行灰度测量后进行统计学处理。
1.9 统计学方法计量数据均采用均数±标准差(Mean±SD)表示,通过Graph Pad Prism 6.0软件采用单因素方差分析进行统计学处理和结果作图,组间比较采用Tukey多重比较,以P < 0.05为差异具有统计学意义。
2 结果 2.1 妊娠大鼠血清AMY和TNF-α水平与SO组比较,APILP组大鼠血清中AMY和TNF-α水平升高,差异具有统计学意义(q= 29.10,q=11.96,P < 0.05)。与APILP组比较,SB组大鼠血清中AMY和TNF-α水平显著下降(q=18.12,q=7.476,P < 0.05),但仍高于SO组(q= 10.98,q=4.49,P < 0.05),见表 1。
| 组别 | AMY(U/L) | TNF-α(pg/mL) | 妊娠大鼠胰腺病理评分 | 胎鼠肺脏病理评分 |
| SO组 | 1, 915.3±452.3 | 107.0±13.3 | 1.56±0.56 | 0.88±0.64 |
| APILP组 | 7, 871.3±623.5a | 193.8±25.4a | 12.44±1.08a | 2.50±0.53a |
| SB组 | 4, 162.1±642.1ab | 139.6±21.1ab | 9.38±1.58ab | 1.63±0.52ab |
| 注:与SO组比较,aP < 0.05,与APILP组比较bP < 0.05 | ||||
光镜下观察可见SO组大鼠胰腺结构清晰,无异常改变; APILP组大鼠胰腺可见腺泡水肿、破裂,小叶结构破坏,间质出血及大量的炎性细胞浸润,其病理评分显著高于SO组(q=26.73,P < 0.05); SB组大鼠胰腺腺泡轻度水肿,少量间质出血及周围组织炎性浸润,其病理评分较APILP组显著降低(q=7.53,P < 0.05),但较SO组仍显著增高(q=19.20,P < 0.05),见表 1。
2.3 胎鼠肺脏病理改变光镜下可见SO组胎鼠肺脏组织结构清晰,膨胀完全,间质无水肿、渗出; APILP组胎肺存在大片肺不张,组织间质增宽、充血水肿,炎症细胞浸润,部分区域内可见肺脏间质及肺泡腔出血,APILP组胎肺组织评分较SO组显著升高(q=8.11,P < 0.05); SB组胎肺组织肺不张显著改善,间质增宽、充血水肿,可见炎症细胞浸润,肺泡腔未见出血,SB组胎肺组织的病理评分显著低于APILP组(q=4.37,P < 0.05),但较SO组显著增高(q=3.74,P < 0.05),见图 1、表 1。
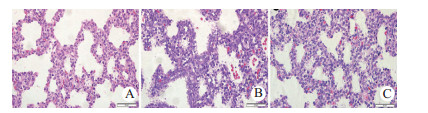
|
| A,B和C分别为SO、APILP和SB组胎鼠肺脏组织(H & E染色,×400) 图 1 各组胎鼠肺脏的病理改变 Fig 1 Morphological changes of fetal lungs in each group |
|
|
NF-κB阳性表达即为视野内细胞浆和(或)核呈黄褐色。SO组胎肺组织中仅少量细胞胞浆内微弱表达NF-κB,APILP组胎肺细胞核内NF-κB呈强阳性表达,SB组胎肺细胞核内NF-κB呈中等阳性表达。高倍视野下APILP组胎肺中NF-κB阳性细胞计数显著高于SO组(q=14.70,P < 0.05),SB组胎肺中NF-κB阳性细胞计数显著低于APILP组(q=6.99,P < 0.05),但仍显著高于SO组(q=7.71,P < 0.05),见图 2、表 2。
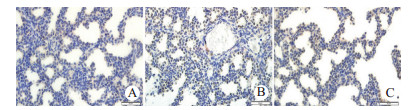
|
| 注:A,B和C分别为SO、APILP和SB组胎鼠肺脏,其中黄褐色细胞即为NF-κB阳性细胞(免疫组织化学染色×400) 图 2 各组胎鼠肺脏中NF-κB的表达情况 Fig 2 NF-κB expression in fetal lungs in each group |
|
|
| 组别 | NF-κB阳性细胞计数 | MPO阳性细胞计数 | p-p38/p38 | TNF-α/actin | ICAM-1/actin | HMGB1/actin |
| SO组 | 29.50±8.80 | 11.75±3.33 | 0.2282±0.0220 | 0.0725±0.0076 | 0.1372±0.0388 | 0.2825±0.0533 |
| APILP组 | 150.63±34.58a | 53.38±8.30a | 0.6367±0.0386a | 0.6313±0.0395a | 0.8958±0.0776a | 0.6478±0.0209a |
| SB组 | 93.00±18.88ab | 27.38±4.75ab | 0.2578±0.0170b | 0.3240±0.0326ab | 0.4177±0.0823 ab | 0.4923±0.0457 ab |
| 注:与SO组比较,aP < 0.05,与APILP组比较bP < 0.05 | ||||||
MPO是中性粒细胞的特异性标志物[16],MPO阳性表达即为视野内胞浆中存在明亮荧光。SO组和SB胎肺内仅有少量MPO阳性细胞,APILP组胎肺内可见大量MPO阳性细胞。高倍视野下APILP组胎鼠肺脏中MPO阳性细胞计数显著高于SO组(q=20.15,P < 0.05),SB组胎肺中MPO阳性细胞计数显著低于APILP组(q=12.58,P < 0.05),但仍显著高于SO组(q=7.56,P < 0.05),见图 3、表 2。

|
| 注:A,B和C分别为SO、APILP和SB组妊娠大鼠的胰腺组织; 胞浆内可见明显绿色荧光的细胞为MPO阳性表达)(免疫荧光染色×200) 图 3 各组胎鼠肺脏中MPO的表达情况 Fig 3 MPO expression in fetal lungs in each group |
|
|
与SO组比较,APILP组胎肺中p-p38MAPK(q=29.74,P < 0.05)、TNF-α(q=37.42,P < 0.05)、ICAM-1(q=21.98,P < 0.05)、HMGB1(q=17.28,P < 0.05)的表达水平显著增加; SB20358干预后,胎肺中p-p38MAPK(q=27.59,P < 0.05)、TNF-α(q=20.58,P < 0.05)、ICAM-1(q=13.85,P < 0.05)和HMGB1(q=7.36,P < 0.05)的表达水平较APILP组显著减少,但除p-p38MAPK外仍高于SO组(q=2.15,P > 0.05;q=16.85,P < 0.05;q=8.13,P < 0.05;q=9.93,P < 0.05),见图 4、表 2。
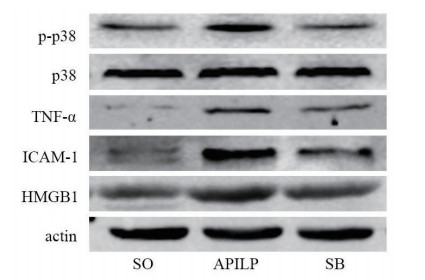
|
| 图 4 各组胎鼠肺脏中p-p38MAPK、p38MAPK、TNF-α、ICAM-1及HMGB1的表达情况 Fig 4 The expression of p-p38MAPK、p38MAPK、TNF-α、ICAM-1and HMGB1in fetal lungs in each group |
|
|
近二十年来,APILP相关孕妇死亡几乎未见报道,但是胎儿病死率可达3.6%~18.75%[5-6, 17],其中4.45%的胎儿存在呼吸窘迫综合征,但其详细机制仍未完全阐明[2]。胰酶在胰腺内异常激活是急性胰腺炎的始发事件,异常激活的胰酶可以引起胰腺和周围组织的自我消化,导致局部炎症反应,甚至全身炎症反应综合征,诱发远隔脏器的功能障碍,而肺脏是最常受累及的器官[18-19]。本研究中,采用胰胆管逆行注射STC建立APILP模型,妊娠大鼠血清内TNF-α、AMY水平显著升高,同时可见妊娠大鼠胰腺和胎鼠肺脏的病理损伤,该结果与前期研究一致[9, 14]。
P38MAPK是一种细胞内丝氨酸/苏氨酸蛋白激酶,可以被多种细胞外理化刺激和多种炎症介质等刺激下磷酸化激活,发挥病理生理作用[10]。p38MAPK和NF-κB信号激活能促进单核/巨噬细胞中多种促炎介质的表达,同时还发现p38MAPK参与了NF-κB的活化,导致急性胰腺炎级联式炎症反应。本研究发现,APILP组胎肺中p38MAPK磷酸化水平显著升高,NF-κB的表达和核转位增多,TNF-α表达显著上调,胎肺的病理评分显著升高; 而应用p38MAPK抑制剂SB203580预处理后,胎肺中p38MAPK磷酸化水平显著降低,NF-κB的表达和核转位减少,TNF-α表达显著下调,胎鼠肺脏的病理损伤显著改善。以上结果表明,p38MAPK磷酸化激活可能进一步激活NF-κB炎症信号通路,参与APILP相关胎鼠肺损伤的病理生理过程; 抑制p38MAPK的活化能够阻断NF-κB炎症信号通路的活化,改善APILP相关胎肺损伤。
中性粒胞细胞在炎症区域中募集,是引起和加重急性胰腺炎时胰腺损伤及导致远隔脏器并发症的中心环节,而中性粒游出与募集需要多种黏附分子的参与,其中ICAM-1介导的中性粒细胞稳定黏附和穿越内皮细胞向炎症部位游出起关键作用[20]。研究表明P38MAPK与NF-κB的活化均可上调ICAM-1的表达[21]。本研究发现,APILP组胎肺中ICAM-1的表达显著升高,胎肺组织中大量中性粒细胞的浸润,而在p8MAPK抑制剂预处理后,胎鼠肺脏组织内ICAM-1的表达水平显著下降,胎肺中中性粒细胞的数量也显著减少。以上结果说明,p38MAPK抑制剂SB203580能够减轻胎鼠肺脏的炎症浸润,这可能与其对ICAM-1表达下调有关。
HMGB1在炎症级联反应的晚期活化,能够激活炎症细胞,诱导炎症介质释放,在急性胰腺炎的发生发展中发挥重要作用[22]。本研究发现,APILP时胎肺中HMGB1水平显著升高,HMGB1可能通过激活胎鼠肺脏中聚集的炎症细胞细胞,诱导炎症介质释放,加重APILP相关胎鼠肺脏损伤。因此,推测SB203580可能是通过降低p38MAPK的磷酸化水平,抑制NF-κB的激活,进而下调HMGB1的表达。
| [2] | Hacker FM, Whalen PS, Lee VR, et al. Maternal and fetal outcomes of pancreatitis in pregnancy[J]. Am J Obstet Gynecol, 2015, 213(4): 568.e1-568.e5. DOI:10.1016/j.ajog.2015.07.031 |
| [3] | Mei FC, Zuo T, Zhao L, et al. Differential JNK, p38 and ERK response to renal injury in a rat model of acute pancreatitis in pregnancy[J]. Arch Gynecol Obstet, 2018, 297(4): 933-942. DOI:10.1007/s00404-018-4668-x |
| [4] | Xu QX, Wang SM, Zhang ZY. A 23-year, single-Center, retrospective analysis of 36 cases of acute pancreatitis in pregnancy[J]. Int J Gynecol Obstet, 2015, 130(2): 123-126. DOI:10.1016/j.ijgo.2015.02.034 |
| [5] | Eddy JJ, Gideonsen MD, Song JY, et al. Pancreatitis in pregnancy[J]. Obstet Gynecol, 2008, 112(5): 1075-1081. DOI:10.1097/aog.0b013e318185a032 |
| [6] | Hernandez A, Petrov MS, Brooks DC, et al. Acute pancreatitis and pregnancy: A 10-year single Center experience[J]. J Gastrointest Surg, 2007, 11(12): 1623-1627. DOI:10.1007/s11605-007-0329-2 |
| [7] | 赵凯亮, 陈辰, 余佳, 等. 罗格利酮对伴高脂血症大鼠重症急性胰腺炎肺损伤的作用研究[J]. 中华急诊医学杂志, 2016, 25(11): 1418-1423. DOI:10.3760/cma.j.issn.1671-0282.2016.11.013 |
| [8] | 崔周军, 王卫星, 赵凯亮, 等. 不同类型GSK-3β抑制剂对重症急性胰腺炎大鼠肾损伤的作用及量效关系[J]. 中华急诊医学杂志, 2018, 27(9): 960-966. DOI:10.3760/cma.j.issn.1671-0282.2018.09.002 |
| [9] | Zhao L, Zuo T, Shi Q, et al. A preliminary study on fetal lung injury in a rat model of acute pancreatitis in pregnancy[J]. Pathol Res Pract, 2017, 213(11): 1370-1377. DOI:10.1016/j.prp.2017.09.016 |
| [10] | Yang ZW, Meng XX, Zhang C, et al. CARD9 gene silencing with siRNA protects rats against severe acute pancreatitis: CARD9-dependent NF-κB and P38MAPKs pathway[J]. J Cell Mol Med, 2017, 21(6): 1085-1093. DOI:10.1111/jcmm.13040 |
| [11] | Tang QQ, Fang MY. Effect of SB203580 on pathologic change of pancreatic tissue and expression of TNF-α and Il-1β in rats with severe acute pancreatitis[J]. Pancreatology, 2013, 13(4): S31-S33. DOI:10.1016/j.pan.2013.07.146 |
| [12] | 樊斌, 石乔, 刘黎明, 等. 氢饱和生理盐水对重症急性胰腺炎大鼠肺损伤的保护作用及对P38MAPK和NF-κB表达的影响[J]. 中华急诊医学杂志, 2015, 24(9): 964-968. DOI:10.3760/cma.j.issn.1671-0282.2015.09.010 |
| [13] | 梅方超, 石乔, 左腾, 等. 巨噬细胞移动抑制因子抑制剂对妊娠晚期大鼠急性坏死性胰腺炎胰腺及胎盘损伤的保护作用及量效关系[J]. 中华急诊医学杂志, 2016, 25(1): 45-49. DOI:10.3760/cma.j.issn.1671-0282.2016.01.011 |
| [14] | Zuo T, Yu J, Wang WX, et al. Mitogen-activated protein kinases are activated in placental injury in rat model of acute pancreatitis in pregnancy[J]. Pancreas, 2016, 45(6): 850-857. DOI:10.1097/mpa.0000000000000528 |
| [15] | Bhatia M. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury[J]. Gut, 2000, 47(6): 838-844. DOI:10.1136/gut.47.6.838 |
| [16] | Malla SR, Kärrman Mårdh C, Günther A, et al. Effect of oral administration of AZD8309, a CXCR2 antagonist, on the severity of experimental pancreatitis[J]. Pancreatology, 2016, 16(5): 761-769. DOI:10.1016/j.pan.2016.07.005 |
| [17] | Geng YX, Li WQ, Sun LQ, et al. Severe acute pancreatitis during pregnancy: eleven years experience from a surgical intensive care unit[J]. Dig Dis Sci, 2011, 56(12): 3672-3677. DOI:10.1007/s10620-011-1809-5 |
| [18] | Wei M. Expression of phosphatidylinositol-3 kinase and effects of inhibitor Wortmannin on expression of tumor necrosis factor- alpha in severe acute pancreatitis associated with acute lung injury[J]. World J Emerg Med, 2015, 6(4): 299. DOI:10.5847/wjem.j.1920-8642.2015.04.009 |
| [19] | 董春阳, 张兴文. 代谢组学在急性胰腺炎中的应用及研究进展[J]. 中华急诊医学杂志, 2019, 28(3): 397-400. DOI:10.3760/cma.j.issn.1671-0282.2019.03.025 |
| [20] | Hong YP, Chen C, Guo WY, et al. Effects of castanospermine on inflammatory response in a rat model of experimental severe acute pancreatitis[J]. Arch Med Res, 2016, 47(6): 436-445. DOI:10.1016/j.arcmed.2016.11.007 |
| [21] | Liu CW, Sung HC, Lin SR, et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway[J]. Sci Rep, 2017, 7: 44689. DOI:10.1038/srep44689 |
| [22] | Yang RK, Tenhunen J, Tonnessen TI. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis[J]. Int J Inflamm, 2017, 2017: 1-6. DOI:10.1155/2017/1817564 |
 2019, Vol. 28
2019, Vol. 28




